Hello everyone! Girls who were in such situations, respond! On May 27th, the first screening was held. Ultrasound was all normal. They recorded the phone just in case, but I did not expect that they could call back, and now a week a call - come back in the CPS, you have a high risk. I do not remember myself, in tears, I got on cotton legs, I took all the pieces of paper. Risk 1:53. The next day I went to double. The ultrasound looked at the stomach for a very long time, and vagnally, he included Doppler several times, and everything seems to be anything, but did not like the Dopplerometry of the truskupidal valve: regurgitation. Entered the data of the new ultrasound in the program and screening results of weekly limitations, the computer issued a risk of SD 1: 6. Sent to genetics. Looking at the conclusion, she explained to me that this regurgitation could be simply a feature of the fetus, but in a charge with an understated indicator PAPP-A - 0.232 MOM it is a marker of chromosomal anomalies. Everything else within the normal range. They suggested passing the biopsy Vorsin Chorion. I still refused, the nurse almost fell from the chair, such as the risk is so tall and ha are not treated and in my place she would not even think of a minute. Interested in genetics about the analysis of the panorama (terribly dear gene. Analysis on maternal blood), she answered me that it could certainly be done, but he excludes only 5 major ha and several very rare, he could not completely exclude an anomalies, and in my case it is recommended invasion. I have already read a ton of articles, questions and everything like this on this topic, and I don't understand what is so terrible found in my analyzes? Regurgitation As it turned out to be physiological on this period and takes place to 18-20 weeks (if it does not speak about the risk of heart defects, many passes after childbirth, and some people live with it and does not affect anything. Especially in her husband prolaps Mintral valve that got from mom, maybe it is somehow interconnected). Hormones may not be indicative at all, because I have been accepted by Duphaston since the beginning of pregnancy, I drone 2 hours before analyzing (it turns out you can not eat for 4 hours before, I didn't say about it), I drank coffee, nervous and worried about the ultrasound and the blood is afraid, and recently chronic fatigue , with the older child I get tired. And all this affects the results. Nothing like the geneticist was not asked, I was not interested, they have some kind of conveyor there, and I seemed to be shoved there for statistics. But the only doubt they planted in me, I drove out, I was worried about a year ahead. Husband persuades a biopsy. I am terribly afraid of the consequences, I'm afraid to lose or harm the child, especially if he is healthy. On the one hand, if everything is fine, sigh with relief and send all doctors away. On the other hand, if everything is bad, what to do? Will I be able to interrupt pregnancy, allow your child inside me, especially now when it seems to me, I begin to feel it. But another option will be able to raise such a child who needs a special approach and a lot of attention, when it sometimes wants to run away from quite a healthy daughter ... Damn, all these thoughts are sent me. I do not know how to be ... just in case I will give screening data:
Term of B-: 13Ned
Heart rate 161 ice / min
Venous duct PI 1,160
Chorion / Planter Low on the front wall
Pupovina 3 vessels
Anatomy of the fetus: everything is determined, everything is normal
b-hgch 1,091 mom
PAPP-A 0.232 MOM
Uterine Arttery PI 1,240 MOM
Trisomy 21 1: 6
Trisomy 18 1: 311
Trisomy 13 1: 205
Preeclampsia to 34 weeks 1: 529
Preeclampsia to 37 weeks 1: 524
7.1. Classification of diabetes
Diabetes(SD) is a group of metabolic diseases characterized by hyperglycemia due to a violation of secretion and / or effectiveness of insulin. Chronic hyperglycemia, developing at the SD, is accompanied by the development of complications by many organs and systems, first of all, from the heart, blood vessels, eyes, kidneys and nerves. A total of 5-6% of the population suffer. In economically developed countries of the world every 10-15 years, the number of patients with CD increases by 2 times. The life expectancy at the CD is reduced by 10-15%.
The causes of the development of the SD are widely varying. In the overwhelming majority of CD cases, either due to absolute insulin deficiency (type 1 diabetes -SD-1), or due to a decrease in the sensitivity of peripheral tissues to insulin in combination with the secreter dysfunction of the pancreatic β-cells (type 2 diabetes -SD-2). In some cases, the assignment of the patient to the SD-1 or SD-2 is difficult, nevertheless, the CD compensation is more significant, and not the exact establishment of its type. The etiological classification allocates four main clinical classes of SD (Table 7.1).
The most common SD-1 (clause 7.5), SD-2 (p. 7.6) and the gestational SD (clause 7.9) are discussed in separate chapters. On the other specific typesthere are only about 1% of cases of SD. The etiology and pathogenesis of these types of SD seems to be more studied compared to SD-1 and especially SD-2. A number of SD variants are due to monogenically inherited genetic defects of the functionβ Bottles.This includes various options for autosomal dominant inherited MODY syndrome (English. maturity Onset Diabetes of the Young- adult diabetes in young), which are characterized by a violation, but not the lack of insulin secretion with normal sensitivity to it peripheral tissues.
Table. 7.1.Classification of diabetes
Casualically rarely found insulin genetic defectsconnected insulin receptor mutation (leprechaunism, Rack Mandehell Syndrome). CD naturally develops with diseases of the exocryan part of the pancreas,leading to the destruction of β-cells (pancreatitis, pancoratectomy, cystic fibrosis, hemochromatosis), as well as under a number of endocrine diseases, in which excessive products of continuity hormones occur (acromegaly, Cushing syndrome). Medicinal preparations and chemicals(Vakor, Pentamidine, Nicotinic acid, diazoxide, etc.) are rarely the cause of SD, but may contribute to the demonstration and decompensation of the disease in individuals with insulin resistance. Row infectious diseases(rubella, cytomegaly, cokes and adenoviral infection) may be accompanied by the destruction of β cells, while most patients define immunogenetic markers SD-1. TO rare forms of immuno-mediated diabetesthe SD, developing in patients with "STIFF-RNAN" -Sindrome (autoimmune neurological disease), as well as a diabetes due to the effects of autoantibodes to insulin receptors. Various variants of SD with increased frequency are found at
many genetic syndromes, in particular, in Down syndromes, Klinfelter, Turner, Tungsten, Prader-Willie and a number of others.
7.2. Clinical aspects of carbohydrate metabolism
Insulinit is synthesized and secreted by β-cells of the islands of Langerhans of the pancreas (PJZ). In addition, the islands of Langerhans secrete glucagon (α-cells), somatostatin (δ cells) and pancreatic polypeptide (PP cell). Hormones of islet cells interact with each other: glucagon normally stimulates insulin secretion, and somatostatin suppresses the secretion of insulin and glucagon. Insulin molecule consists of two polypeptide chains (A-chain - 21 amino acid; in-chain - 30 amino acids) (Fig. 7.1). Insulin synthesis begins with the formation of preproinsulin, which splits protease to education proinsulin.In the secretory granules of the Machine, Golgi, Pinsulin splits into insulin and C-peptide,which are released into the blood in the process of exocytosis (Fig. 7.2).
The main stimulant of insulin secretion is glucose. The release of insulin in response to increasing blood glucose two-phase(Fig. 7.3). The first, or acute phase lasts a few minutes, and it is associated with the release of accumulation
 Fig. 7.1.The diagram of the primary structure of the insulin molecule
Fig. 7.1.The diagram of the primary structure of the insulin molecule
 Fig. 7.2.Insulin biosynthesis scheme
Fig. 7.2.Insulin biosynthesis scheme
shegne in an β-cell insulin in the period between meals. The second phase continues until the level of glycemia reaches normal merchant (3.3-5.5 mmol / l). Similarly, the β-cells affect the preparations of sulfonylurea.
According to the portal insulin system reaches liver- its main target organ. Hepatic receptors bind half of the secreted hormone. Another half, falling into systemic bloodstream, reaches muscles and adipose tissue. Most of the insulin (80%) is subjected to proteolytic decay in the liver, the rest is in the kidneys, and only a minor amount is metabolized directly with muscle and fat cells. Norma PJZ.
 Fig. 7.3.Two-phase insulin release under the influence of glucose
Fig. 7.3.Two-phase insulin release under the influence of glucose
adult man secretes 35-50 units per day, which is 0.6-1.2 units per 1 kg of body weight. This secretion is divided into nutritional and basal. Food secretioninsulin CO consults the postprandial lifting level of glucose, i.e. Due to it, neutralization of hyperglycimizing foods is ensured. The amount of food insulin approximately corresponds to the number of carbohydrates taken - about 1-2.5
by 10-12 g of carbohydrates (1 bread unit - hee). Basal secretion insulinprovides the optimal level of glycemia and anabolism in the intervals between food and during sleep. Basal insulin is secreted at a speed of about 1 un / h, with long-term exercise or long starvation, it decreases significantly. Food insulin accounts for at least 50-70% of the daily production of insulin (Fig. 7.4).
Insulin secretion is subject not only to food, but also daily
 Fig. 7. .4.
Daily Production Insulin Norma
Fig. 7. .4.
Daily Production Insulin Norma
oscillations:the need for insulin rises in the early morning hours, and in the future gradually falls during the day. Thus, 2.0-2,5 sneakers are secreted for breakfast on 1 heb, for lunch - 1.0-1.5 units, and for dinner - 1.0 units. One of the reasons for such a change in insulin sensitivity is a high level of a number of conjunral hormones (primarily cortisol) in the morning hours, which gradually drops to the minimum at the beginning of the night.
Basic physiological effects insulinthere are stimulation of glucose transfer through insulin-dependent tissue membranes. The main bodies of insulin are liver, adipose tissue and muscle. To insulin-dependent tissues, the flow of glucose into which does not depend on the effects of insulin, primarily include the central and peripheral nervous system, the endothelium of blood vessels, blood cells, etc. Insulin stimulates the synthesis of glycogen in the liver and muscles, the synthesis of fats in the liver and adipose tissue, synthesis Proteins in the liver, muscles and other organs. All these changes are directed to the utilization of glucose, which leads to a decrease in its level in the blood. Physiological insulin antagonist is glucagonwhich stimulates the mobilization of glycogen and fats from the depot; Normally, the level of glucagon changes the reciprocal insulin products.
Insulin biological effects are mediated by receptorswhich are located on target cells. Insulin receptor is a glycoprotein consisting of four subunits. With a high level of insulin in the blood, the number of its receptors on the principle of lower regulation is reduced, which is accompanied by a decrease in cell sensitivity to insulin. After binding insulin with a cellular receptor, the complex comes inside the cell. Next inside the muscle and fat cell, insulin causes mobilization of intracellular vesicles, which contain glucose conveyorGLUT-4. As a result, the vesicles move to the cell surface, where GLUT-4 performs the function of the inlet for glucose. A similar effect on GLUT-4 has a physical exertion.
7.3. Lab diagnostics and criteria for compensation of diabetes
Laboratory diagnostics of the SD is based on the determination of blood glucose level, while the criteria for diagnostics are united for all
types and variants of the SD (Table 7.2). The data of other laboratory studies (the level of glucosuria, the definition of the level of the glycated hemoglobin) should not be used to verify the diagnosis of the diagnosis. The diagnosis of SD can be set on the basis of two-time detection of one of three criteria:
1. With obvious SD symptoms (polyuria, polydipsy) and glucose level in solid capillary blood, more than 11.1 mmol / l, regardless of the time of day and preceding meals.
2. At the level of glucose in a solid capillary blood, an empty stomach of more than 6.1 mmol / l.
3. At the level of glucose in solid capillary blood 2 hours after receiving 75 grams of glucose (oral glucose-bearing test) more than 11.1 mmol / l.
Table. 7.2.Criteria for diagnosing diabetes
 The most important and significant test in the diagnosis of the CD is to determine the level of glycemia on an empty stomach (minimum 8 hours of fasting). In the Russian Federation, the level of glycemia is usually estimated in solid blood. Many countries are widely used to determine the level of glucose
The most important and significant test in the diagnosis of the CD is to determine the level of glycemia on an empty stomach (minimum 8 hours of fasting). In the Russian Federation, the level of glycemia is usually estimated in solid blood. Many countries are widely used to determine the level of glucose
in blood plasma. Oral glucose-bearded test(OGTT; Determination of the level of glucose 2 hours after administration inside the 75 grams of glucose dissolved in water) in this regard, there is a smaller value. Nevertheless, on the basis of OGTT is diagnosed glucose tolerance violation(NTG). NTH is diagnosed if the level of solid capillary blood glykemia is not exceeded 6.1 mmol / l, and 2 hours after the load, glucose is higher than 7.8 mmol / l, but below 11.1 mmol / l. Another embodiment of carbohydrate exchange is violated glycemia on an empty shop(NGN). The latter is established if the level of glycemia of solid capillary blood is in an empty stomach in the range of 5.6-6.0 mmol / l, and 2 hours after the load with glucose less than 7.8 mmol / l). NTG and NGNT are currently united by the term prediabetsince both categories of patients are highly high risk of manifestation of SD and the development of diabetic macroangiopathy.
To diagnose the SD, the level of glycemia must be determined by standard laboratory methods. In the interpretation of glycemia indicators, it should be borne in mind that an empty stomach level of glucose in solid venous blood corresponds to its level in a solid capillary. After receiving food or OGTT, its level in venous blood is about 1.1 mmol / l lower than in capillary. The glucose content in the plasma is about 0.84 mmol / l higher than in solid blood. In order to assess the compensation and adequacy of the CD therapy, the level of glycemia is estimated in capillary blood using portable glucometerpatients themselves, their relatives or medical personnel.
With any type of diabetes, as well as with a significant load of glucose can develop glucosuriawhich is a consequence of excess of the glucose reabsorption threshold from the primary urine. The glucose reabsorption threshold significantly varies (≈ 9-10 mmol / l). As a separate glucosuria indicator for the diagnosis of the SD should not be used. Normally, with the exception of cases of significant food load refined carbohydrates, glucosuria is not found.
Products ketone tel(Acetone, acetoacetate, β-hydroxybutyrate) is significantly intensified at an absolute insulin deficiency. When decompensation, SD-1 may define pronounced ketonuria(Explores the test strips, which are lowered in the urine). Easy (trace) ketonuria can be determined in healthy people with starvation and a fermented diet.
An important laboratory indicator, which is used for the differential diagnosis of types of SD, as well as to identify the formation of insulin deficiency in patients with SD-2, is the level C-peptide.In terms of the level of C-peptide in the blood, it is indisputable to judge the inserting ability of the β-cells of the PJZ. The latter produce proinsulin, from which the C-peptide is cleaved before secretion, which falls into the blood in the same amounts with insulin. Insulin is 50% contacted in the liver and has half-life in peripheral blood about 4 minutes. C-peptide from blood flow liver is not removed and has a half-life in the blood of about 30 minutes. In addition, it is not associated with cell receptors on the periphery. Therefore, the definition of the C-peptide level is a more reliable test to estimate the function of the insular apparatus. The level of C-peptide is most informatively examined against the background of stimulation samples (after eating or administration of glucagon). The test is non-informative if it is carried out against the background of a pronounced decompensation of SD, since the pronounced hyperglycemia has a toxic effect on β-cells (glucosotoxicity). Insulin therapy for several preceding days to the test results will not affect.
Main the purpose of treatmentany type of CD is to prevent its late complications, which can be achieved against the background of its stable compensation on a number of parameters (Table 7.3). The main criterion for the quality of compensation for carbohydrate metabolism under the CD is the level glycated (glycosylated) hemoglobin (HBA1C).The latter is hemoglobin, unknownly associated with glucose. In the erythrocytes of glucose comes independently of insulin, and the glycosylation of hemoglobin is an irreversible process, and its degree is directly proportional to the concentration of glucose with which it has contacted for 120 days of its existence. A small part of hemoglobin is glycosylated and normal; With the CD, it can be significantly increased. The HBA1C level, in contrast to the level of glucose, which is constantly changing, integrally reflects the glycemia over the past 3-4 months. It is with this interval that the HBA1C level is recommended to assess the compensation of the SD.
Chronic hyperglycemia is far from the only risk factor for the development and progression of late complications of the SD. Concerning evaluation of compensation SDbased on the complex
laboratory and instrumental research methods (Table 7.3). In addition to indicators characterizing the condition of carbohydrate metabolism, the level of blood pressure and lipid spectrum of blood are the most important.
Table. 7.3.Sugar Diabetes Compensation Criteria
 In addition to the above compensation criteria, an individual approach is needed when planning the objectives of the CD treatment. The probability of development and progression of late complications of SD (especially microangiopathy) increases with an increase in the duration of the disease. Thus, if in children and young patients, the length of the diabetes in the future can reach several decades, it is necessary to achieve optimal glycemia indicators, then in patients who have a CD manifest in elderly and old age, rigid euglecemic compensation, significantly improving the risk of hypoglycemia, Not always appropriate.
In addition to the above compensation criteria, an individual approach is needed when planning the objectives of the CD treatment. The probability of development and progression of late complications of SD (especially microangiopathy) increases with an increase in the duration of the disease. Thus, if in children and young patients, the length of the diabetes in the future can reach several decades, it is necessary to achieve optimal glycemia indicators, then in patients who have a CD manifest in elderly and old age, rigid euglecemic compensation, significantly improving the risk of hypoglycemia, Not always appropriate.
7.4. Insulin and insulin therapy preparations
Insulin preparations are vital to patients with SD-1; In addition, they receive up to 40% of patients with SD-2. To common indications for the appointment of insulin therapy at SD,many of whom actually overlap one other include:
1. Type 1 diabetes
2. Panketectomy
3. Ketoacidotic and hyperosmolar coma
4. With diabetes mellitus type 2:
Explicit signs of insulin deficiency, such as the progressiveness of body weight and ketosis, expressed hyperglycemia;
Large surgical interventions;
Acute macro-complicated complications (stroke, myocardial infarction, gangrene, etc.) and severe infectious diseases, accompanied by decompensation of carbohydrate metabolism;
The level of glycemia is an empty stomach of more than 15-18 mmol / l;
The lack of payment of compensation, despite the prescription of the maximum daily doses of various tableted saccharincing drugs;
Late stages of late complications of SD (severe polyneuropathy and retinopathy, chronic renal failure).
5. The inability to make compensation of gestational diabetes using diet and therapy.
By origininsulin preparations can be classified into three groups:
Animals insulins (pork);
Human insulins (semi-synthetic, genetic engineering);
Analogs of insulins (Lizpro, Aspart, Glargin, Demide).
Progress of technologies for the production of human insulins led to the use of pork insulin(different from human one amino acid) has recently decreased significantly. Pork insulin can be used for the production of human insulin semi-synthetic methodwhich implies the replacement of one different amino acid in its molecule. The highest quality is different genetically engineeringhuman insulins. To obtain them, the man's genome site responsible for insulin synthesis is associated with genome E.Coli.or yeast culture, as a result of which the latter begin to produce human insulin. Creature analogs Insulinwith the help of permutations of various amino acids, the purpose of obtaining drugs with the given and most favorable pharmacokinetics was pursued. So, Insulin Lizpro (Humalog) is analogue
insulin of ultrashort action, while its saccharincing effect develops after 15 minutes after injection. An analogue of Glargin insulin (Lantus), on the contrary, is characterized by a long action that continues throughout the day, while the feature of the drug kinetics is the lack of pronounced peaks of the plasma concentration. Most of the currently used insulin preparations and its analogues are produced in concentration100 U / ml. By duration of actioninsulines are divided into 4 main groups (Table 7.4):
Table. 7.4.Pharmacokinetics of drugs and insulin analogs
 1.
Ultrashort Action (Lizpro, Aspart).
1.
Ultrashort Action (Lizpro, Aspart).
2. Short action (simple human insulin).
3. The average duration of action (insulins at the neutral protamine Hagedorn).
4. Long-term action (glagragin, detech).
5. Mixtures of insulins of various duration of action (Novomix-30, Humulin-MH, Humalog mix-25).
Preparations ultrashort action[Lizpro (Humalog), aspart (Novorad)] are analogs of insulin. Their advantages are the rapid development of the sugar effect effect after the injection (after 15 minutes), which allows you to make an injection immediately before meals or even immediately after eating, as well as a short duration of action (less than 3 hours), which reduces the risk of hypoglycemia. Preparations short action(Simple insulin, insulin-regular) is a solution containing insulin at a concentration of 100 units / ml. Injection of simple insulin is made 30 minutes before meals; The duration of action is about 4-6 hours. The preparations of ultra-screw and short action can be administered subcutaneously, intramuscularly and intravenously.
Among drugs average duration of actionmost often preparations are used at the neutral protamine Hagedorn (NPH). NPH is a protein that is unknown adsorb insulin, slowing down its suction from the subcutaneous depot. The effective duration of the Action of NPH insulins is usually about 12 hours; They are entered only subcutaneously. Insulin NPH is a suspension, in connection with which, in contrast to simple insulin in the vial, it is muddy, and with a long standing there is a suspension, which must be thoroughly mixed before injection. NPH insulins Unlike other preparations of prolonged action can be mixed with a short-acting insulin (simple insulin), while the pharmacokinetics of the mixture components will not change, since the NPH will not bind additional amounts of simple insulin (Fig. 7.5). In addition, Protamin is used to prepare standard mixes of insulin analogs (Novomix-30, Humalog-Mix-25).
Among the drugs of long-term action are currently actively using analogues of insulin glargin(Lantus) and detemerary(Leewemir). The favorable feature of the pharmacokinetics of these drugs is that, in contrast to the Insulins of the NPC, they provide a more uniform and long-term flow of the drug from the subcutaneous depot. In this regard, Glargin can be appointed only once a day, while almost no time regardless of the time of day.
 Fig. 7.5.Pharmacokokinetics of various insulin preparations:
Fig. 7.5.Pharmacokokinetics of various insulin preparations:
a) monocomponent; b) standard insulin mixtures
In addition to monocomponent drugs insulin, clinical practice are widely used standard mixtures.As a rule, we are talking about short or ultrashort insulin mixtures with insulin of the average duration of action. For example, the drug "Humulin-MW" contains in one bottle of 30% of simple insulin and 70% insulin NPH; The drug "Novomiks-30" contains 30% insulin aspart and 70% of the Crystal Protamine Suspension of Insulin ASPART; The drug "Humalog-Mix-25" contains 25% insulin Lyspro and 75% prothnce suspension of insulin leasing. Advantage
standard insulin mixtures is the replacement of two injections of one and several large accuracy of the mixture components; The disadvantage is the impossibility of individual dosing of the individual components of the mixture. This determines the preference of the use of standard insulin mixtures for CD-2 therapy or with the so-called traditional insulin therapy(appointment of fixed doses of insulins), whereas for intensive insulin therapy(Flexible dose selection depending on the indicators of glycemia and the amount of carbohydrates in food) is preferable to the use of monocomponent drugs.
The key to successful insulin therapy is a clear observance injection techniques.There are several ways to introduce insulin. The easiest and easiest of the reliable method - injection with insulin syringe.An more convenient way to introduce insulin are injections using syringe knobswhich is a combined device containing an insulin tank (cartridge), a dosing system and an injector needle.
For supporting therapy (when it comes to a pronounced decompensation of SD or about critical states), insulin is introduced subcutaneously. Injection of the insulin of a short action is recommended to do into subcutaneous fatty tissue of the abdomen, insulin of prolonged action - into the fiber of the hip or shoulder (Fig. 7.6 a). Injections are made deep into subcutaneous tissue through widely compressed skin at an angle of 45 ° (Fig. 7.6 b). The patient needs to recommend a daily change of insulin injection sites within the same area in order to prevent the development of lipodystrophs.
TO factors affecting insulin absorption speedfrom the subcutaneous depot, the insulin dose should be attributed (the increase in the dose increases the duration of absorption), the injection site (absorption is faster from the abdominal fiber), the ambient temperature (heating and massage of the injection site accelerates absorption).
A more complex administration method that, nevertheless, in many patients allows you to achieve good treatment results, is the use of insulin dispenseror systems for continuous subcutaneous insulin administration. The dispenser is a portable device consisting of a computer that sets the insulin supply mode, as well as an insulin supply system, carried out on a catheter and a miniature needle into subcutaneous
 Fig. 7.6.Injection injections: a) typical injection places; b) the position of the needle of the insulin syringe during injection
Fig. 7.6.Injection injections: a) typical injection places; b) the position of the needle of the insulin syringe during injection
fat tissue. With the help of the dispenser, a continuous basal introduction of a short or ultrashort insulin (the rate of about 0.5-1 e / hour) is carried out, and before taking food, depending on the content of carbohydrates and the level of glycemia, the patient introduces the necessary bolus dose of the same insulin of a short action. The advantage of insulin therapy with the help of the dispenser is the introduction of an insulin of a short (or even ultrashort) action, which in itself is somewhat more physiologically, since the absorption of prolonged insulin preparations is exposed to large fluctuations; In this regard, the continuous introduction of a short action insulin turns out to be a more manageable process. The disadvantage of insulin therapy with the help of the dispenser is the need for a constant carrying device, as well as the long-term foundation of an injection needle in the subcutaneous tissue, which requires periodic control over the process of supplying insulin. Insulin therapy with the help of a dispenser is primarily shown to patients with SD-1, which are ready to master the technique of its maintenance. Especially in this regard, you should pay attention to patients with a pronounced phenomenon of "Morning Dawn", as well as for pregnant women and planning pregnancy patients with SD-1 and Paris
ents with a disordered way of life (the possibility of a more flexible power mode).
7.5. Type 1 diabetes
SD-1 - Organship-specific autoimmunethe disease leading to the destruction of insulin production β-cells of PJZ islets, manifested by an absolute insulin deficiency. In some cases, patients with explicit SD-1 lack autoimmune lesion markers of β-cells (idiopathic SD-1).
Etiology
SD-1 is a disease with hereditary predisposition, but its contribution to the development of the disease is small (determines its development by about 1 / s). The concordancy of single-person twins on SD-1 is only 36%. The probability of development of SD-1 in a child with a sick mother is 1-2%, father - 3-6%, brother or sister - 6%. Some or a few humoral markers of autoimmune lesions of β-cells, to which antibodies include antibodies to PJZ, antibodies to glutamate decarboxylase (Gad65) and antibodies to tyrosine phosphatase (IA-2 and ια-2β) are found in 85-90% of patients. . Nevertheless, cell immunity factors are attached to the destruction of β cells. SD-1 is associated with HLA haplotypes such as DQAand DQB,at the same time alone allele HLA-DR / DQmay be predisposing to the development of the disease, while others are protest. With an increased frequency of SD-1, combined with other autoimmune endocrines (autoimmune thyroiditis, Addison disease) and non-alcohol diseases, such as alopecia, vitiligo, crown disease, rheumatic diseases (Table 7.5).
Pathogenesis
SD-1 manifests during the destruction of an autoimmune process 80-90% β-cells. The speed and intensity of this process can vary significantly. Most often typical flowdiseases in children and young people this process proceeds quite quickly, followed by a violent manifestation of the disease, in which the appearance of the first clinical symptoms to the development of ketoacidosis (up to the ketoacidotic coma) can pass only a few weeks.
Table. 7.5.Type 1 diabetes
 Continuation of table. 7.5.
Continuation of table. 7.5.
 In other, significantly more rare cases, as a rule, in adults over 40 years old, the disease can flow latent (Latent autoimmune diabetes adults - LADA),at the same time, in the debut of the disease, such patients often establishes a diagnosis of SD-2, and over the course of several years, the CD compensation can be achieved by the appointment of sulfonylurea drugs. But in the future, usually 3 years later, there are signs of absolute insulin deficiency (weight loss, ketonuria, expressed hyperglycemia, despite the reception of tableted saccharincing drugs).
In other, significantly more rare cases, as a rule, in adults over 40 years old, the disease can flow latent (Latent autoimmune diabetes adults - LADA),at the same time, in the debut of the disease, such patients often establishes a diagnosis of SD-2, and over the course of several years, the CD compensation can be achieved by the appointment of sulfonylurea drugs. But in the future, usually 3 years later, there are signs of absolute insulin deficiency (weight loss, ketonuria, expressed hyperglycemia, despite the reception of tableted saccharincing drugs).
The basis of the pathogenesis of SD-1, as indicated, is the absolute insulin deficiency. The impossibility of entering glucose into insulin-dependent fabrics (fat and muscular) leads to energy failure resulting in the intensifies lipolysis and proteolysis, with which the loss of body weight is associated. The increase in the level of glycemia causes hyperosmolarity, which is accompanied by osmotic diuresis and pronounced dehydration. Under conditions of insulin deficiency and energy failure, conjunral hormone products are being developed (glucagon, cortisol, growth hormone), which, despite increasing glycemia, determines the stimulation of glukegenesis. The increase in lipolysis in adipose tissue leads to a significant increase in the concentration of free fatty acids. With insulin deficiency, the liposynthetic ability of the liver is suppressed, and
fatty acids begin to turn on in ketogenesis. The accumulation of ketone bodies leads to the development of diabetic ketosis, and in the future - ketoacidosis. With the progressive increase in dehydration and acidosis, a comatose state is developing (see paragraph 7.7.1), which, in the absence of insulin therapy and rehydration, inevitably ends with death.
Epidemiology
On SD-1, there are about 1.5-2% of all cases of diabetes, and this relative figure will continue to decrease due to the rapid growth of the incidence of SD-2. The risk of developing SD-1 throughout the life of the representative of the White Race is about 0.4%. The incidence of SD-1 increases by 3% per year: by 1.5% due to new cases and another 1.5% due to an increase in the life expectancy of patients. The prevalence of SD-1 varies depending on the ethnic composition of the population. For 2000, it amounted to 0.02% in Africa, 0.1% in South Asia, as well as in South and Central America and 0.2% in Europe and North America. The most high incidence of SD-1 in Finland and Sweden (30-35 cases per 100 thousand population per year), and the lowest in Japan, China and Korea (respectively 0.5-2.0 cases). The age-related peak of the manifestation of SD-1 corresponds to about 10-13 years. In the overwhelming majority of cases, SD-1 manifests up to 40 years.
Clinical manifestations
IN typical casesespecially in children and young people, SD-1 debuts a bright clinical picture, which develops for several months or even weeks. The manifestation of SD-1 can provoke infectious and other concomitant diseases. Characteristic common for all types of SD symptoms,related with hyperglycemia: polydipsy, polyuria, skin itch, but at SD-1 they are very pronounced. So, throughout the day, patients can drink and extract up to 5-10 liters of fluid. Specificfor SD-1, the symptom, which is due to the absolute deficiency of insulin, is weight loss, reaching 10-15 kg for 1-2 months. It is characterized by severe general and muscular weakness, reduced performance, drowsiness. At the beginning of the disease, some patients may occur an increase in appetite, which is replaced by anorexia as the ketoacidosis develops. The latter is characterized by the appearance of the smell of acetone (or fruit odor) from the mouth, Tosh
nota, vomiting, often pain in the stomach (pseudoperitonite), severe dehydration and ends with the development of a comatose state (see paragraph 7.7.1). In some cases, the first manifestation of SD-1 in children is a progressive disorder of consciousness up to coma against the background of concomitant diseases, as a rule, infectious or acute surgical pathology.
In relatively rare cases of the development of SD-1 in persons over 35-40 years (Latent autoimmune adult diabetes)the disease can be manifest not so bright (moderate polydipsy and polyuria, lack of body weight loss) and even reveal by chance with routine definition of the level of glycemia. In these cases, the patient often establishes a diagnosis of SD-2 and tableted saccharincing preparations (TSP) are prescribed, which for some time provide acceptable CD compensation. Nevertheless, for several years (often during the year), the patient appears symptoms caused by the growing absolute deficit of insulin: weight loss, the impossibility of maintaining normal glycemia on the background of TSP, ketosis, ketoacidosis.
Diagnostics
Given that SD-1 has a bright clinical picture, and is also a relatively rare disease, the screening definition of the level of glycemia in order to diagnose SD-1 is not shown. The probability of the development of the disease near the nearest relatives of patients is low, which, together with the lack of effective methods of primary prophylaxis, SD-1 determines the inexpractingness of the study of immunogenetic disease markers. The diagnosis of SD-1 in the overwhelming majority is based on the identification of significant hyperglycemia in patients with severe clinical manifestations of the absolute deficiency of insulin. OGTT for the purpose of diagnostics SD-1 has to be carried out very rarely.
Differential diagnosis
In doubtful cases (detection of moderate hyperglycemia in the absence of explicit clinical manifestations, manifestation in a relatively elderly), as well as for the purpose of differential diagnostics with other types of SD, is used to determine the level C-peptide(basal and 2 hours after meals). Indirect diagnostic value in doubtful cases may have definition immunological markersSD-1 - Antibodies to Oral
PJZ, to glutamatdekarboxylase (Gad65) and tyrosine phosphatase (Ia-2 and Ia-2β). Differential diagnosis of SD-1 and SD-2 is presented in Table. 7.6.
Table. 7.6.Differential diagnosis and differences between SD-1 and SD-2
 Treatment
Treatment
Treatment of any type of CD is based on three basic principles: Sakharosyncing therapy (with SD-1 - insulin therapy), diet and patient training. Insulinotherapyat SD-1 wears substitutionand its goal is the maximum imitation of the physiological products of the hormone in order to achieve adopted compensation criteria (Table 7.3). To the physiological secretion of insulin is the most approximate intensive insulin therapy.The need for insulin corresponding to it basal secretion,it is provided by two insulin injections of the average duration of the action (in the morning and evening) or one long-acting insulin injection (Glargy). Total dose of basal inso-
lina should not exceed half of the entire daily need for the preparation. Food or Bolus Insulin Secretionit is replaced by the injections of the insulin of a short or ultrashort action before each meal intake, while its dose is calculated, based on the amount of carbohydrates, which is supposed to be taken during the upcoming food intake, and the existing level of glycemia determined by the patient with a glucometter before each insulin injection (Fig. 7.7 ).
Approximate intensive insulin therapy scheme,which will vary almost every day, can be represented as follows. They proceed from the fact that the daily need for insulin is about 0.5-0.7 units per 1 kg of body weight (for a patient with a body weight of 70 kg about 35-50 units). About 1 / s - 1/2 of this dose will be insulin of prolonged action (20-25 units), 1/2 - 2 / s Insulin of short or ultrashort action. The dose of insulin NPH is divided into 2 injections: in the morning 2 / s of its dose (12 units), in the evening - 1 / s (8-10 units).
Purpose the first stagesealing insulin therapy is the normalization of the level of glucose on an empty stomach. The evening dose of Insulin NPH is usually introduced at 22-23 hours, Morning along with the injection of a short action insulin in front of breakfast. When selecting an evening dose of insulin NPH, it is necessary to keep in mind the possibility of developing a number
 Fig. 7.7.Scheme of intensive insulin therapy
Fig. 7.7.Scheme of intensive insulin therapy
quite typical phenomena. The cause of the morning hyperglycemia may be insufficient dose of insulin prolonged action, because by the morning the need for insulin increases significantly (The "Morning Dawn" phenomenon).In addition to dose deficiency, its excess can lead to morning hyperglycemia. phenomenon Somoga(Somogyi), postgoglycemic hyperglycemia. This phenomenon is explained by the fact that the maximum tissue sensitivity to insulin is marked between 2 and 4 hours of the night. It is at this time that the level of basic conjunral hormones (cortisol, growth hormone, etc.) is normal. If the evening dose of insulin of prolonged action is excessive, then at this time develops hypoglycemia.Clinically, it can manifest themselves a bad sleep with nightmarish dreams, unconscious actions in a dream, morning headache and a breakdown. Development at this time of hypoglycemia causes a significant compensatory glucagon compensatory emission and other conjunral hormones followed hyperglycemia in the morning clock.If in this situation, it is not reduced, but to increase the dose of prolonged insulin, invested in the evening, night hypoglycemia and morning hyperglycemia will be exacerbated that in the end it can lead to the syndrome of chronic insulin syndrome (Somoga syndrome), which is a combination of obesity with chronic decompensation of SD, frequent Hypoglycemia and progressive late complications. For the diagnosis of the phenomenon of Somoga, it is necessary to study the level of glycemia about 3 h, which is an integral component of the selection of insulin therapy. If the decline in the night hypoglycemia is accompanied in terms of the development of night hypoglycemia, is accompanied by hyperglycemia in the morning (the phenomenon of the morning dawn), the patient needs to recommend an earlier rise (6-7 in the morning), while insulin introduced overnight continues to maintain the normal level of glycemia.
The second injection of the Insulin NPH is usually done before breakfast together with the morning injection of the insulin of a short (ultrashort) action. In this case, the dose is selected primarily on the basis of the level of glycemia in front of the main daytime meals (lunch, dinner); In addition, it can limit the development of hypoglycemia in the intervals between meals, for example, at noon, between breakfast and lunch.
All Dose Insulin prolonged action(Glargin) is introduced once a day, while not fundamentally, at what time. Kinetics
glargin's insulins and detech are more favorable in terms of the risk of hypoglycemia, including night.
A short or ultrashort insulin dose Even in the first patient, insulin destination will depend on the amount of carbohydrates used (bread units) and the level of glycemia before injection. Conditionally, based on the daily rhythm of insulin secretion, about 1/4 of the short-acting insulin (6-8 units) is given to dinner, the remaining dose of approximately equally divided into breakfast and lunch (10-12 units). The higher the initial level of glycemia, the less it will decrease by the unit of inserted insulin. Injection of a short action insulin is made 30 minutes before meals, ultrashort action immediately before meals or even immediately after eating. The adequacy of a short-acting insulin dose is estimated in terms of glycemia 2 hours after meals and before the next meal.
To calculate the insulin dose with intensive insulin therapy, it is sufficiently counting the number X, based on the carbohydrate component. At the same time, not all carbohydrate products are taken into account, but only the so-called calculated. The latter includes potatoes, grain products, fruits, liquid dairy and sweet products. Products containing undervaluable carbohydrates (most vegetables) are not taken into account. Special exchange tables have been developed, with the help of which, expressing the amount of carbohydrates in He, you can calculate the necessary dose of insulin. One XE corresponds to 10-12 g of carbohydrates (Table 10.7).
After taking food containing 1 x, the level of glycemia increases by 1.6-2.2 mmol / l, i.e. Approximately as much as the glucose level is reduced when Introduction of 1 U. insulin. In other words, on each hee contained in the food, which is planned to eat, must be introduced in advance (depending on the time of day) about 1 units of insulin. In addition, we need to record the results of self-control of the level of glycemia, which is performed before each injection, and the time of day (about 2 units of insulin on 1 heb in the morning and at lunch, 1 units on 1 x - for dinner). So, if hyperglycemia is revealed, the insulin dose, calculated in accordance with the upcoming meal (in terms of the number of xE), should be increased, and vice versa, if hypoglycemia is revealed, insulin is introduced less.
Table. 7.7.Equivalent replacement of products constituting 1 x
 For example, if a patient is 30 minutes to the planned dinner containing 5 hehe, the level of glycemia is 7 mmol / l, it must be introduced 1 units to the glycemia to decrease to a normal level: from 7 mmol / l to about 5 mmol / l. In addition, 5 Uzinulin must be introduced on the coating 5 x. Thus, the patient in this case will introduce 6 units of a short or ultrashort action.
For example, if a patient is 30 minutes to the planned dinner containing 5 hehe, the level of glycemia is 7 mmol / l, it must be introduced 1 units to the glycemia to decrease to a normal level: from 7 mmol / l to about 5 mmol / l. In addition, 5 Uzinulin must be introduced on the coating 5 x. Thus, the patient in this case will introduce 6 units of a short or ultrashort action.
After the manifestation of the SD-1 and the start of insulin therapy, for quite a long time, the need for insulin may be small and be less than 0.3-0.4 units / kg. This period is indicated as a phase of remission, or "Honeymoon".After the hyperglycemia and ketoacidosis period, which suppress insulin secretion is 10-15% by preserved β-cells, compensation of hormonal-metal disorders by the introduction of insulin restores the function of these cells, which then assume the provision of an insulin body at a minimum level. This period can continue from several weeks to several years, but ultimately, due to the autoimmune destruction of the remaining β-cells, the "honeymoon" ends.
Dietwith SD-1 in trained patients who own the skills of self-control and selection of insulin dose, may be liberalized, i.e. approaching free. If the patient has no excess or body weight deficit, the diet must be
isocalorian. The main component of food at SD-1 is carbohydrates, which should have about 65% of the daily calorage. Preference should be given to products containing complex, slowly suction carbohydrates, as well as products rich in food tissue. Products containing carbohydrates (flour, sweet), should be avoided. The proportion of proteins should be reduced to 10-35%, which helps to reduce the risk of developing microangiopathy, and the share of fats - up to 25-35%, and the limit fats should account for up to 7% of the calorage, which reduces the risk of atherosclerosis. In addition, it is necessary to avoid taking alcoholic beverages, especially strong.
An integral component of working with a patient with SD-1 and a pledge of its effective compensation is training of patients.Throughout life, the patient must daily independently depend on numerous factors to change the dose of insulin. Obviously, it requires possession of certain skills that the patient needs to be trained. "Patient SD-1 School" is organized in endocrinological hospitals or outpatient and represents 5-7 structured classes, on which a doctor or a specially trained nurse in interactive mode using various visual benefits conducts patient training principles selfontrol.
Forecast
In the absence of insulin therapy, the SD-1 patient inevitably dies from the ketoacidotic coma. With inadequate insulin therapy, against the background of which the CD compensation criteria are not achieved and the patient is in a state of chronic hyperglycemia (Table 7.3), late complications (§ 7.8) begin to develop and progress. With SD-1, the greatest clinical significance in this regard has manifestations of diabetic microdgium (nephropathy and retinopathy) and neuropathy (diabetic foot syndrome). Macroangiopathy with SD-1 on the foreground is relatively rare.
7.6. Type 2 diabetes
Type 2 diabetes- a chronic disease manifesting a violation of carbohydrate exchanges with the development of hyperglycemia due to insulin resistance and secretory β-cell dysfunction,
as well as lipid metabolism with the development of atherosclerosis. Since the main cause of the death and disability of patients is complications of systemic atherosclerosis, SD-2 is sometimes referred to as a cardiovascular disease.
Table. 7.8.Type 2 diabetes
 Etiology
Etiology
SD-2 is a multifactorial disease with hereditary predisposition. Concordability on SD-2 in single-time twins reaches 80% or more. Most patients with SD-2 indicate the presence of SD-2 for the nearest relatives; In the presence of SD-2 in one of the parents, the likelihood of its descendant during the life is 40%. Which one gene, whose polymorphism determines the predisposition to SD-2, was not detected. The factors of the environment play great importance in the implementation of hereditary predisposition to SD-2. SD-2 Risk Risk factors are:
Obesity, especially visceral (see paragraph 11.2);
Ethnicity (especially when changing the traditional lifestyle on Western);
Sedentary lifestyle;
Features of the diet (high consumption of refined carbohydrates and low fiber content);
Arterial hypertension.
Pathogenesis
Pathogenetically SD-2 is a heterogeneous group of metabolic disorders, it is precisely that determines its significant clinical heterogeneity. The basis of its pathogenesis is insulin resistance (decrease in mediated insulin of glucose utilization by tissues), which is implemented against the background of the secretory dysfunction of β-cells. Thus, there is a violation of the balance of sensitivity to insulin and insulin secretion. Secretor dysfunctionβ - fleetit is to slow down the "early" secretory emission of insulin in response to an increase in blood glucose. At the same time, the 1st (fast) secretion phase, which lies in the emptying of vesicles with the accumulated insulin, is actually absent; The 2nd (slow) secretion phase is carried out in response to the stabilizing hyperglycemia constantly, in tonic mode, and despite the excessive secretion of insulin, the level of glycemia against the background of insulin resistance is not normalized (Fig. 7.8).
The consequence of hyperinsulinemia is to reduce the sensitivity and number of insulin receptors, as well as suppression
post-receptor mechanisms encouraging insulin effects (insulin resistance).The content of the main conveyor of glucose in muscle and fat cells (GLUT-4) is reduced by 40% in persons with visceral obesity and 80% in persons with SD-2. Due to the insulin resistance of hepatocytes and portal hyperinsulinemia, glucose hyperproduction, liver,and the hyperglycemia is developing, which is detected in most patients with SD-2, including in the early stages of the disease.
The hyperglycemia itself adversely affects the nature and level of secretory activity of β-cells (glucosotoxicity). For a long time, over the years and decades, the existing hyperglycemia ultimately leads to depletion of insulin products β-cells and some symptoms may appear in the patient. insulin deficiency- Slimming, ketosis with associated infectious diseases. However, the residual insulin products, which is sufficient to prevent ketoacidosis, is almost always preserved at SD-2.
Epidemiology
SD-2 defines the epidemiology of the SD as a whole, since it accounts for about 98% of cases of this disease. The prevalence of SD-2 varies in different countries and ethnic groups. In European
 Fig. 7.8.Secretor dysfunction of β-cells with type 2 diabetes mellitus (loss of the 1st fast insulin secretion phase)
Fig. 7.8.Secretor dysfunction of β-cells with type 2 diabetes mellitus (loss of the 1st fast insulin secretion phase)
countries, USA and the Russian Federation, it is about 5-6% of the population. With age, the incidence of SD-2 increases: among adults the prevalence of SD-2 is 10%, among people over 65 reaches 20%. The incidence of SD-2 is 2.5 times higher among the indigenous people of America and the Hawaiian Islands; Among the Indians of the Pima tribe (Arizona) it reaches 50%. Among the rural population of India, China, Chile and African countries that lead the traditional lifestyle, the prevalence of SD-2 is very low (less than 1%). On the other hand, among the immigrants in the Western industrial countries it reaches a significant level. So, among the immigrants from India and China, living in the United States and the UK, the prevalence of SD-2 reaches 12-15%.
WHO predicts an increase in the number of diabetes patients in the world by 122% over the next 20 years (from 135 to 300 million). This is due to both the progressive aging of the population and with the distribution and aggravation of the urbanized lifestyle. In recent years, there has been a significant "rejuvenation" of SD-2 and the growth of its incidence among children.
Clinical manifestations
In most cases, pronounced clinical manifestations are absent,and the diagnosis is established during the routine definition of the level of glycemia. The disease usually manifests over the age of 40 years, while the overwhelming majority of patients has obesity and other components of metabolic syndrome (see paragraph 11.2). Patients do not impose complaints about working capacity if there are no other reasons for this. Complaints of thirst and polyuria rarely achieve considerable severity. Quite often patients are concerned about the skin and vaginal itching, and therefore they turn to dermatologists and gynecologists. Since many years (on average, about 7 years), many patients at the time of detection of the disease in the clinical picture, at the time of the identification of the disease in the clinical picture, are often dominated from the actual manifestation of the SD-2 before diagnosis. symptoms and manifestations of late complications of SD.Moreover, the first appeal of the patient with SD-2 for medical help is very often due to late complications. So, patients can be hospitalized in surgical hospitals with peptic lesions of the legs (diabetic foot syndrome),contact linking progressive vision to ophthalmologists (diabetic retinopathy),hospitalized with heart attacks, stroke
tami, oblique the defeat of the foot vessels in the institution, where they first find hyperglycemia.
Diagnostics
Diagnostic criteria, uniform for all types of SD, are presented in clause 7.3. The diagnosis of SD-2 in the overwhelming majority is based on the detection of hyperglycemia in individuals with typical clinical signs of SD-2 (obesity, age over 40-45 years, positive family history of SD-2, other components of metabolic syndrome), in the absence of clinical and laboratory signs Absolute insulin deficiency (pronounced weight loss, ketosis). The combination of the high prevalence of SD-2, characteristic of a long asymptomatic flow and the possibility of preventing his heavy complications, subject to early diagnosis, predetermine the need screeningthose. Examination in order to exclude SD-2 among individuals without any symptoms of the disease. The main test, as indicated, is the definition the level of glycemia is an empty stomach.It is shown in the following situations:
1. All people are over the age of 45, especially in excess of body weight (CMT more than 25 kg / m 2) at intervals every 3 years.
2. In a smallest age in the presence of an excess body weight (BMI with more than 25 kg / m 2) and additional risk factors to which include:
Sedentary lifestyle;
SD-2 for the nearest relatives;
Belonging to the national risk of development of SD-2 (African Americans, Latin Americans, Indigenous Americans, etc.);
Women who gave birth to a child weighing more than 4 kg and / or in the presence of gestational diabetes as a history;
Arterial hypertension (≥ 140/90 mm Hg);
Level of HDL\u003e 0.9 mmol / l and / or triglycerides\u003e 2.8 mmol / l;
Polycystic ovarian syndrome;
NTG and NGN;
Cardiovascular diseases.
Significant increase in the incidence of SD-2 among children dictates the need for screening definition of the level of glycemia among children and adolescents(starting from 10 years with an interval of 2 years or with the beginning
pubertata, if he occurred at an earlier age) belonging to the groups of increased risk to which children belong with abundance of body weight(BMI and / or body weight\u003e 85 percentile, appropriate age, or weight of more than 120% in relation to the ideal) in combination with any two listed additional risk factors:
SD-2 among the relatives of the first or second line of kinship;
Belonging to high-risk nationalities;
Clinical manifestations associated with insulin resistance (Acanthosis Nigricans,arterial hypertension, dyslipidemia);
SD, including gestational, mother.
Differential diagnosis
The differential diagnosis of SD-2 and SD-1, the principles of which are described in paragraph 7.5 are described in paragraph 7.5 (Table 7.6). As indicated, in most cases it is based on the data of the clinical picture. In cases where the establishment of the type of CD meets difficulties, or there is a suspicion of some rare variant of the SD, including in the framework of hereditary syndromes, the most important practical question to which it is necessary to answer is whether the patient needs a patient in insulin therapy.
Treatment
The main components of the treatment of SD-2 are: diet therapy, extension of physical activity, sugar therapy, prevention and treatment of late CD complications. Since most patients with SD-2 suffer obesity, the diet should be aimed at reduced weight (hypochalorial) and the prevention of late complications, primarily macroangiopathy (atherosclerosis). Hypolarian dietit is necessary for all patients with excess body weight (BMI 25-29 kg / m 2) or obesity (BMI\u003e 30 kg / m 2). In most cases, it should be recommended to reduce the daily edge of food to 1000-1200 kcal for women and up to 1200-1600 kcal for men. The recommended ratio of the main food components at SD-2 is similar to that with SD-1 (carbohydrates - 65%, proteins 10-35%, fats up to 25-35%). Use alcoholit is necessary to limit due to the fact that it is an essential source of additional calories, in addition, the admission of alcohol on the background of the tera
fDI with sulfonylurea and insulin can provoke the development of hypoglycemia (see paragraph 7.7.3).
Recommendations for expanding physical activitymust be individualized. At the beginning, aerobic loads (walking, swimming) of moderate intensity duration of 30-45 minutes 3-5 times a day (about 150 minutes per week) are recommended. In the future, it is necessary to gradually increase physical exertion, which significantly helps to reduce and normalize body weight. In addition, physical exertion helps to reduce insulin resistance and have a hypoglycimizing effect. The combination of dietotherapy and extension of physical exertion without the appointment of sugar drugs makes it possible to maintain SD compensation in accordance with the established objectives (Table 7.3) approximately 5% of patients with SD-2.
Preparations for sugarinizing therapywhen SD-2 can be divided into four main groups.
I. Preparations contributing to the reduction of insulin resistance (sensitizers).This group includes metformin and thiazolidindions. Metforminis the only one currently used by the drug from the group biguanids.The main components of the mechanism of its action are:
1. Suppression of gluconeogenesis in the liver (decrease in glucose products with liver), which leads to a decrease in the level of glycemia on an empty stomach.
2. Reducing insulin resistance (increase in glucose disposal by peripheral tissues, primarily with muscles).
3. Avnaeobic glycolysis activation and reduction of glucose suction in the small intestine.
Metforminit is a preparation of the first choice of sugar therapy in patients with SD-2, obesity and hyperglycemia on an empty stomach. The initial dose is 500 mg per night or during dinner. In the future, the dose gradually increases to 2-3 grams for 2-3 receptions. Among the side effects relatively often there are dyspeptic phenomena (diarrhea), which, as a rule, transient and pass independently after 1-2 weeks of receiving the drug. Since metformin does not have a stimulating effect on insulin products, on the background of monotherapy by this drug hypoglycemia
develop (its action is designated as antihyperglycemic, and not as hypoglycemic). Contraindications to the appointment of metformin are pregnancy, severe heart, hepatic, renal and other organ failure, as well as the hypoxic states of another genesis. An extremely rare complication, which occurs when appointing metformin without taking into account the presented contraindications is lactatacidosis, which is a consequence of anaerobic glycolysis hyperactivation.
Thiazolidindions(Pioglitazone, Rosigtyazon) are agonists of γ-receptors activated by peroxiz (PPAR-γ). Thiazolidindions activate the metabolism of glucose and lipids in muscle and adipose tissues, which leads to an increase in the activity of endogenous insulin, i.e. To eliminate insulin resistance (insulin sensitizers). The daily dose of pioglitazone is 15-30 mg / day, roseglitazone - 4-8 mg (per 1-2 reception). The combination of thiazolidindiones with Metformin is very effective. The contraindication to the purpose of thiazolidindion is an increase (2.5 times or more) level of hepatic transaminases. In addition to hepatotoxicity, the side effects of thiazolidine edion include the delay in fluid and swelling, which are more often developing during a combination of insulin preparations.
II. Preparations affectingβ - Better and contributing to strengthening the secretion of insulin.This group includes sulfonylurea and clay preparations (prandial glykemia regulators), which are used mainly to normalize the level of glycemia after eating. Main target preparations of sulfonylmochevines(PSM) are β-cells of pancreatic islands. PSM binds to β-cell membrane with specific receptors. This leads to the closure of ATP-dependent potassium channels and depolarization of the cell membrane, which in turn contributes to the opening of calcium channels. The flow of calcium inside β-cell leads to their degranulation and insulin emission into blood. In clinical practice, quite a lot of PSMs are used, which differ in the duration and severity of the sugar effect (Table 7.9).
Table. 7.9.Preparations of sulfonylmochevines
 The main and fairly frequent side effect of PSM is hypoglycemia (see paragraph 7.7.3). It can occur with the drug overdose, its cumulation (renal failure),
The main and fairly frequent side effect of PSM is hypoglycemia (see paragraph 7.7.3). It can occur with the drug overdose, its cumulation (renal failure),
non-compliance with the diet (passing meals, alcohol intake) or mode (significant physical exertion, before which the dose of PSM is not reduced or carbohydrates are not taken).
To group hinides(Prandial regulators of glycemia) repaglinide(benzoic acid derivative; daily dose 0.5-16 mg / day) and nateglinida(D-phenylalanine derivative; daily dose of 180-540 mg / day). After taking drugs, the preparations quickly and reversible with the sulfonylurevine receptor on the β cell, as a result of which a short increase in insulin level occurs, which mimics the first phase of its secretion is normal. Preparations are accepted in 10-20 minutes to the main meals, usually 3 times a day.
III. Preparations that reduce glucose absorption in the intestine.
This group includes the akaboz and guar resin. The mechanism of action of the acarbosis is the reversible blockade of α-glycosidases of the small intestine, as a result of which the processes of sequential fermenting and suction of carbohydrates slow down, the speed of resorption and glucose admission to the liver decreases and the level of postprandial glycemia is reduced. The initial dose of acarbosis is 50 mg 3 times a day, in the future, the dose can be increased to 100 mg 3 times a day; The drug is accepted immediately before eating or during food. The main side effect of acarbosa is intestinal dyspepsia (diarrhea, flatulence), which is associated with the receipt of non-discovered carbohydrates into the colon. The sacrarization effect of the acarbosis is very moderate (Table 7.10).
In clinical practice, tableted saccharincing drugs are effectively combined with each other and with insulin preparations, since most patients are simultaneously defined as mercular and postprandial hyperglycemia. There are numerous fixed combinationspreparations in one tablet. Most often in one tablet, metformin with various PSMs, as well as metformin with thiazolidinediones, is combined.
Table. 7.10.The mechanism of action and the potential efficiency of tableted sugar drugs
 IV. Insulins and analogs of insulins
IV. Insulins and analogs of insulins
At a certain stage, insulin preparations begin to receive up to 30-40% of patients with SD-2. Indications for insulin therapy at SD-2 are presented at the beginning of paragraph 7.4. The most common option for the translation of patients with SD-2 on insulin therapy is to assign insulin of prolonged action (insulin NPH, Glargin or Detech) in combination with the received tableted sugar-based drugs. In a situation where the level of glycemia is not possible to control the appointment of metformin or the last contraindicated, the patient is assigned to the evening (overnight) insulin injection. If it is impossible to control with the help of tabletaic drugs as a mercury and postprandial glycemia, the patient is translated into monoinsulinatherapy. Usually, with SD-2 insulin therapy is carried out on the so-called "Traditional" scheme,which implies the appointment of fixed doses of insulin prolonged and short action. In this plan
the standard mixtures of insulins containing a short (ultrashort) and prolonged action in one bottle are convenient. The choice of traditional insulin therapy is determined by the fact that under SD-2, it is often appointed to elderly patients whose learning to an independent change in insulin dose is difficult. In addition, intensive insulin therapy, the purpose of which is to maintain the compensation of carbohydrate exchange at the level approaching the normoglycemia, is increasing the risk of hypoglycemia. If for young patients, light hypoglycemia do not represent a serious danger, in elderly patients with a reduced threshold of the sensation of hypoglycemia, they can have very unfavorable effects from the cardiovascular system. Young patients with SD-2, as well as patients in terms of effective learning opportunities, an intensive version of insulin therapy can be appointed.
Forecast
The main reason for the disability and death of patients with SD-2 is the late complications (see paragraph 7.8), most often diabetic macroangiopathy. The risk of developing certain late complications is determined by the complex of factors that are discussed in the relevant chapters. A universal risk factor for their development is chronic hyperglycemia. Thus, the decrease in the level of HBA1C in patients with SD-2 per 1% leads to a decrease in total mortality by about 20%, by 2% and 3% - by about 40%, respectively
7.7. Acute complications of diabetes
7.7.1. Diabetic ketoacidosis
Diabetic ketoacidosis (DCA)- Decompensation of SD-1, due to the absolute deficiency of insulin, in the absence of timely treatment of an ending ketoacidotic coma (QC) and death.
Etiology
The cause of DCA is an absolute insulin deficiency. This or that severity of the DCA is determined in most patients at the time of the manifestation of SD-1 (10-20% of all DCA cases).
The patient with a diagnosed diagnosis of SD-1 DCA can develop when the insulin is stopped, often by the patient itself (13% of DCA cases), on the background of concomitant diseases, primarily infectious, in the absence of an increase in insulin dose
Table. 7.11.Diabetic ketoacidosis
 Up to 20% of DC-1 development cases in young patients with SD-1 are associated with psychological problems and / or disorders of food behavior (fear of weight gain, fear of hypoglycemia, teenage problems). Quite frequent cause of DCA in a number of countries is
Up to 20% of DC-1 development cases in young patients with SD-1 are associated with psychological problems and / or disorders of food behavior (fear of weight gain, fear of hypoglycemia, teenage problems). Quite frequent cause of DCA in a number of countries is
cancel of insulin by the patient himself due to the high cost of drugs for some sectors of the population (Table 7.11).
Pathogenesis
At the heart of the pathogenesis of DCA lies the absolute deficiency of insulin in combination with the increase in the products of conjunral hormones, such as glucagon, catecholamines and cortisol. As a result, there is a significant increase in the products of glucose liver and a disturbance of its disposal by peripheral tissues, the increase in hyperglycemia and the disruption of the osmolarity of the extracellular space. Insulin deficiency in combination with a relative excess of contrincing hormones at DCA leads to a release of free fatty acids (lipolysis) and their uncompressed oxidation in the liver to ketone bodies (β-hydroxybutyrate, acetoacetate, acetone), as a result of which hypercohememia develops, and in the future Metabolic acidosis. As a result of pronounced glucose, osmotic diuresis develops, dehydration, sodium loss, potassium and other electrolytes (Fig. 7.9).
Epidemiology
The frequency of new cases of DCA is 5-8 per 1000 patients with SD-1 per year and directly depends on the level of the organization of medical care patients with SD. Every year in the United States occurs about 100,000 hospitalizations about DCA, while taking into account the costs of one patient for hospitalization of 13 thousand dollars, more than $ 1 billion per year is spent annually on stationary treatment of DCA. In the Russian Federation in 2005, DKA was recorded in 4.31% of children, 4.75% of adolescents and 0.33% of adult patients with SD-1.
Clinical manifestations
Depending on the cause of the cause, depending on the cause caused by its reason, can take from several weeks to day. In most cases, DCA is preceded by diabetes decompensation symptoms, but sometimes they may not have time to develop. Clinical symptoms of DCA include polyuria, polydipsy, slimming, spilled abdominal pain ("Diabetic pseudoperithonite"), dehydration, pronounced weakness, smell of acetone from mouth (or fruit smell), gradually clouding consciousness. True coma at DCA has recently been developing relatively rarely due to early diagnosis. In physical research, signs of dehydration are detected: reduction
 Fig. 7.9. Pathogenesis of ketoacidotic coma
Fig. 7.9. Pathogenesis of ketoacidotic coma
turgor of the skin and density of eyeballs, tachycardia, hypotension. In emerging cases, the breathing of Kussmouul develops. More than 25% of patients with DCA develop vomiting, which can resemble coffee thick on color.
Diagnostics
It is based on the clinical picture data, indications of the presence of the patient SD-1, as well as the data of the laboratory study. For DCA, hyperglycemia is characterized (in some cases, insignificant), ketonuria, metabolic acidosis, hyperosmolarness (Table 7.12).
Table. 7.12.Laboratory diagnostics of sharp complications of diabetes
 When examining patients with acute decompensation of the SD, it is necessary to determine the level of glycemia, creatinine and urea, electrolytes, on the basis of which effective osmolarity is calculated. In addition, an escort and ground state is needed. Effective osmolarity(EO) is calculated according to the following formula: 2 *. Norma EO is 285 - 295 mosm / l.
When examining patients with acute decompensation of the SD, it is necessary to determine the level of glycemia, creatinine and urea, electrolytes, on the basis of which effective osmolarity is calculated. In addition, an escort and ground state is needed. Effective osmolarity(EO) is calculated according to the following formula: 2 *. Norma EO is 285 - 295 mosm / l.
Most patients with DCA are determined leukocytosisthe severity of which is proportional to the level of ketone bodies in the blood. Level sodiumas a rule, it is reduced due to osmotic fluid outflow from intracellular spaces to extracellular in response to hyperglycemia. Less often sodium level can be reduced falsely as a result of a pronounced hyper-
triglyceridemia. Level potassiumserum can be initially elevated due to its movement from extracellular spaces.
Differential diagnosis
Other reasons for the loss of consciousness in patients with SD. Differential diagnosis with hyperosmolar coma, as a rule, does not cause difficulties (develops in elderly patients with SD-2) and does not have a large clinical value, because The principles of treatment of both states are similar. If it is impossible to quickly find out the reason for the loss of the patient's consciousness with the SD, the introduction of glucose is shown, since Hypoglycemic states are found much more often, and a quick positive dynamics against the background of the introduction of glucose itself allows us to find out the reason for the loss of consciousness.
Treatment
Treatment of DKA implies rehydration, correction of hyperglycemia, electrolyte disorders, as well as treatment of diseases that caused diabetes decompensation. Treatment is most optimally carried out in the resuscitation department of a specialized medical institution. In adult patients without severely concomitant heart pathology, still in a pre-hospital stage as a primary measure for the purpose of rEYDRATEMENTit is recommended to introduce an isotonic solution (0.9% NaCl) approximately with the speed of a liter per hour (about 15-20 ml per kilogram of weight per hour). A complete reimbursement of a fluid deficiency, which with DCA is 100-200 ml per kg of weight, must be achieved within the first days of treatment. With concomitant heart or renal failure, this period of time must be increased. For children, the recommended volume of isotonic solution for rehydration therapy is 10-20 ml per kg of body weight per hour, and in the first 4 hours it should not exceed 50 ml per kg of weight. Full rehydration is recommended to achieve approximately 48 hours. After in the background of parallel insulin therapy, the level of glycemia will decrease in about 14 mmol / l, go to the transfusion of a 10% glucose solution, which continues to rehydration.
Currently adopted the concept of "small doses" insulinin the treatment of DCA. Only a short action insulin is used. The most optimal use of intravenous insumption
lina. The intramuscular introduction of insulin, which is less efficient, possibly only with moderate severity of the DCA, with stable hemodynamics and if impossible for intravenous therapy. In the latter case, injections are made in the straight muscle of the abdomen, while the needle for intramuscular injections is put on an insulin syringe (for a reliable intramuscular hit), and on this needle, insulin is recruited from the bottle into the syringe.
There are several options for intravenous insulin administration. First, insulin can be introduced "into the rubber band" of the infusion system, while the required amount of insulin is gained into an insulin syringe, after which 1 ml of isotonic solution is obtained. Up to reaching the level of glycemia, 14 mmol / l is an hourly patient introduced 6-10 units of short action; further (In parallel with the change of rehydration solution with isotonic by 10% glucose)depending on the time of the definable indicators of glycemia, the dose of insulin is reduced to 4-8 units per hour. The recommended reduction rate of the level of glycemia should not exceed 5 mmol / l per hour. Another embodiment of intravenous insulin therapy implies the use of perfuzor. For the preparation of the perpetuator, 2 ml of a 20% solution of the human albumin solution is added to the 50 mg of 0.9% isotonic solution. If the intramuscular path of the insulin administration is selected, 20 units of a short action is initially introduced initially, after which it is 6 units, and after reaching the level of glycemia, 14 mmol / l dose is reduced to 4 units per hour. After complete stabilization of hemodynamics and compensation for acid-base disorders, the patient is translated into subcutaneous insulin injections.
As indicated, despite the significant potassium deficiencyin the body (overall loss of 3-6 mmol / kg), with DCA its level before the start of insulin therapy can be somewhat elevated. Nevertheless, the start of transfusion of the potassium chloride solution is recommended to be carried out simultaneously with the beginning of insulin therapy, if the plasma potassium level is less than 5.5 mmol / l. Successful correction of potassium deficiency occurs only against the background of the pH normalization. With a low pH, potassium intake is significantly reduced significantly, in connection with this, if possible, the dose of overflowing potassium chloride is desirable to adapt to a specific pH indicator (Table 7.13).
Table. 7.13.Potassium deficit correction scheme
 * For calculation, the following data uses:
* For calculation, the following data uses:
1 g KCl \u003d 13.4 mmol; 1 mmol KCl \u003d 0.075 g. In a 4% solution of KS1: in 100 ml - 4 g KS1, in 25 ml - 1 g of KS1, in 10 ml of 0.4 g of KS1.
The reason for decompensation of diabetes is often infectious diseases(pyelonephritis, infected ulcer with diabetic foot syndrome, pneumonia, sinusitis, and so on.). There is a rule according to which, with DCA antibiotic therapy, it is prescribed to almost all patients with a subfebilitation or fever, even in the absence of a visible focus of infection, since actually for the DCA, an increase in body temperature is not typical.
Forecast
DCA mortality is 0.5-5%, while most cases are due to late and unskilled medical care. Mortality is highest (up to 50%) among older patients.
7.7.2. Hyperosmolar Coma
Hyperosmolar Coma(GOK) - a rare acute complication of SD-2, developing due to pronounced dehydration and hyperglycemia Against the absence of an absolute insulin deficiency, accompanied by high mortality (Table 7.14).
Etiology
GOK, as a rule, develops in elderly patients with SD-2. Such patients are most often lonely, live without care, neglected by their condition and self-control and make not enough liquid. Often, infection (diabetic foot syndrome, pneumonia, acute pyelonephritis), brain disorders lead to decompensation
circulatory and other conditions, as a result of which patients are badly moving, do not take sugar-based drugs and liquid.
Table. 7.14.Hyperosmolar Coma (GOK)
 Pathogenesis
Pathogenesis
The increasing hyperglycemia and osmotic diuresis determine the pronounced dehydration, which on the reasons specified above is not filled from the outside. The result of hyperglycemia and dehydration is the plasma hyperosmolarity. An integral component of the Pathogenesis of the GOK is the relative deficiency of insulin and the excess of the counter-pole hormones, nonetheless, which persists at SD-2, the residual secretion of insulin is sufficient to suppress lipolyase and ketogenesis, as a result of which the ketoacidosis does not occur.
In some cases, moderate induction of acidosis may be determined as a result of hyperlactatemia against the background of tissue hypoperfusion. With pronounced hyperglycemia to preserve the osmotic balance in the cerebrospinal fluid, the sodium content coming from the cells of the brain increases where potassium gets in exchange. The transmembrane potential of nervous cells is disturbed. The progressive perisage of consciousness in combination with convulsive syndrome is developing (Fig. 7.10).
Epidemiology
The GOK accounts for 10-30% of acute hyperglycemic states in adults and elderly patients with SD-2. Approximately 2/3 of the cases of the GOK develops in persons with undiagnosed to this dia.
Clinical manifestations
The features of the clinical picture of the hyperosmolar coma are:
A complex of signs and complications of dehydration and hypoperfusion: thirst, dryness of mucous membranes, tachycardia, arterial hypotension, nausea, weakness, shock;
Focal and generalized convulsions;
Fever, nausea and vomiting (40-65% of cases);
Of the concomitant diseases and complications, thrombosis of deep veins are often found, pneumonia, brainwater disorders, gastroparesis.
Diagnostics
It is based on the data of the clinical picture, the age of the patient and the history of SD-2, pronounced hyperglycemia in the absence of ketonuria and ketoacidosis. Typical laboratory signs of GOK are presented in Table. 7.12.
 Fig. 7. .10.
Pathogenesis of hyperosmolar coma
Fig. 7. .10.
Pathogenesis of hyperosmolar coma
Differential diagnosis
Other acute states developing in patients with diabetes, most often with concomitant pathology leading to a pronounced decompensation of SD.
Treatment
Treatment and monitoring in the GOK, with the exception of some features, do not differ from those described for the ketoacidotic diabetic coma (clause 7.7.1):
Larger amount of initial rehydration of 1.5-2 liters per 1st hour; 1 L - for the 2nd and 3rd hour, then 500 ml / h isotonic solution of sodium chloride;
The need for the introduction of potassium-containing solutions is usually greater than with a ketoacidotic coma;
Insulin therapy is similar to that with the CC, but the need for insulin is less and the level of glycemia must be reduced no faster than 5 mmol / l per hour to avoid the development of brain edema;
The introduction of a hypotonic solution (NaCl 0.45%) is better to avoid (only with pronounced hypernatremia:\u003e 155 mmol / l and / or efficient osmolarity\u003e 320 mos / l);
In the introduction of bicarbonate there is no need (only in specialized resuscitation compartments with acidosis with pH< 7,1).
Forecast
Mortality at the GOK is high and is 15-60%. The worst prognosis in elderly patients with severely accompanying pathology, which is often the cause of decompensation of SD and the development of the GOK.
7.7.3. Hypoglycemia
Hypoglycemia- Reducing the level of glucose in blood serum (<2,2- 2,8 ммоль/л), сопровождающее клинический синдром, характеризующийся признаками активации симпатической нервной системы и/или дисфункцией центральной нервной системы. Гипогликемия как лабораторный феномен не тождественен понятию «гипогликемическая симптоматика», поскольку лабораторные данные и клиническая картина не всегда совпадают.
Etiology
An overdose of insulin preparations and its analogues, as well as sulfonylurea drugs;
Disadvantage of food on the background of unchanged sugar therapy;
Acceptance of alcoholic beverages;
Physical exertion on the background of unchanged sugar therapy and / or without additional carbohydrate reception;
Development of late complications of SD (autonomous neuropathy with gastroparesis, renal failure) and a number of other diseases (adrenal insufficient failure, hypothyroidism, liver failure, malignant tumors) with unchanged sugar therapy (continuation of the reception and cumulation of TSPs on the background of renal failure, preservation of the former insulin dose);
Violation of insulin administration (intramuscular injection instead of subcutaneous);
Artificial hypoglycemia (conscious overdose of sugar drugs by the patient himself);
Organic hyperinsulinism - insulin (see paragraph 10.3).
Pathogenesis
The pathogenesis of hypoglycemia is to violate the balance between the flow of glucose into the blood, its disposal, the level of insulin and the conjunral hormones. Normally, at the level of glycemia, within 4.2-4.7 mmol / l, the products and release of insulin from β-cells are suppressed. Reducing the level of glycemia less than 3.9 mmol / l is accompanied by stimulation of conjunral hormone products (glucagon, cortisol, growth hormone, adrenaline). Neuroglycopenic symptoms develops with a decrease in the level of glycemia less than 2.5-2.8 mmol / l. For overdose insulinand / or drugs sulfonylmochinahypoglycemia is developing due to the direct hypoglycimizing effect of an exogenous or endogenous hormone. In the case of overdose by the preparations of sulfonylurea, hypoglycemic symptoms may repeatedly recur after the binding of the attack due to the fact that the duration of the range of drugs can reach the day or more. TSP, which do not have a stimulating effect on insulin products (metformin, thiazolidindions), the hypoglycemia themselves cannot be caused by themselves, but when they add sulfonylurea or insulin, the reception of the latter in the same dose may cause hypoglycemia due to the cumulation of the shah-surging effect of combination therapy (Table . 7.15).
Table. 7.15.Hypoglycemia
 Ending table. 7.15
Ending table. 7.15
 When receiving alcoholit occurs the suppression of glukegenesis in the liver, which is the most important factor opposing hypoglycemia. Physical exerciseprotect the insulin-dependent glucose utilization, due to which there are causes of hypoglycemia on the background of unchanged sugar-sacrament therapy and / or in the absence of additional reception of carbohydrates.
When receiving alcoholit occurs the suppression of glukegenesis in the liver, which is the most important factor opposing hypoglycemia. Physical exerciseprotect the insulin-dependent glucose utilization, due to which there are causes of hypoglycemia on the background of unchanged sugar-sacrament therapy and / or in the absence of additional reception of carbohydrates.
Epidemiology
Light, quickly bubble hypoglycemia in patients with SD-1, receiving intensive insulin therapy, can develop several times a week, and relatively harmless. Per patient who is in intensive insulin therapy, per year accounts for 1 case of severe hypoglycemia. In most cases, hypoglycemia are developing at night. With SD-2 in 20% of patients receiving insulin, and in 6% receiving sulfonylurea preparations, for at least one episode of severe hypoglycemia develops for at least one episode.
Clinical manifestations
Two main groups of symptoms are distinguished: adrenergic, associated with the activation of the sympathetic nervous system and emission of adrenaline adrenalines, and neuroglycopenic, associated with the impaired functioning of the central nervous system against the background of the deficit of its main energy substrate. TO adrenergicsymptoms include: tachycardia, mydriasis; anxiety, aggressiveness; shiver, cold sweat, paresthesia; nausea, strong hunger, hypersalization; Diarrhea, abundant urination. TO neuroglycopenicsymptoms include asthenia,
reducing the concentration of attention, headache, feeling of fear, confusion, disorientation, hallucinations; Speech, visual, behavioral disorders, amnesia, violation of consciousness, convulsions, transient paralysis, to whom. A clear dependence of the severity and sequence of development of symptoms as hypoglycemia may not be. Only adrenergic or only neuroglycopenic symptoms may occur. In some cases, despite the restoration of normoglycemia and continuing therapy, patients can be in a sliding or even comatose state over several hours and even days. Long-term hypoglycemia or its frequent episodes can lead to irreversible changes in the central nervous system (primarily in the cortex of large hemispheres), the manifestations of which are significantly varied from delicious and hallucinatory-paranoid episodes to typical epileptic seizures, which is the inevitable outcome of which are resistant dementia.
Hyperglycemia is subjectively transferred to patients lighter than episodes even light hypoglycemia. Therefore, many patients due to the fear of hypoglycemia consider it necessary to maintain glycemia at a relatively high level, which actually corresponds to the decompensation of the disease. Overcoming this stereotype requires some considerable efforts of doctors and training personnel.
Diagnostics
The clinical picture of hypoglycemia in a patient with a SD in combination with laboratory (as a rule, with a glucose meter) detecting a low blood glucose level.
Differential diagnosis
Other reasons leading to loss of consciousness. If the cause of the loss of the consciousness of the patient of the SD is unknown and it is impossible to conduct express analysis of the level of glycemia, it shows the introduction of glucose. Often there is a need to find out the causes of the development of frequent hypoglycemia in patients with SD. Most often they are a consequence of inadequate sugar-based therapy and a low-level patient's knowledge of their disease. It should be remembered that to reduce the need for sugar therapy until its complete cancellation ("disappeared SD") can lead a number of diseases (adrenal insufficiency, hypothyroidism, renal and liver failure), including malignant tumors.
Treatment
For the treatment of light hypoglycemia, in which the patient is conscious and may assist himself, it is usually enough to take food or a liquid containing carbohydrates in the amount of 1-2 bread units (10-20 g of glucose). Such a number is contained, for example, in 200 ml of sweet fruit juice. Drinks more effectively stop hypoglycemia, because in the liquid form glucose is significantly absorbed. If symptoms continues to grow, despite the continued reception of carbohydrates, it is necessary to intravenous administration of glucose or intramuscular glucagon. In the same way, severe hypoglycemia is treated with loss of consciousness. In this case, the patient is introduced about 50 ml 40% glucose solution intravenously.The introduction of glucose must be continued until the binding of the attack and the normalization of glycemia, although more dose is up to 100 ml and more, as a rule, is not required. Glucagonit is introduced (as a rule, prepared in the factory conditions filled with a syringe) intramuscularly or subcutaneously. After a few minutes, the level of glykemia due to the induction of glucoenolysis glucagon is normalized. However, this is not always happening: with a high level of insulin in blood glucagon is ineffective. The half-life of glucagon is shorter than insulin. With alcoholism and liver diseases, the synthesis of glycogen is disturbed, and the administration of glucagon can be ineffective. A side effect of glucagon administration may be vomiting that creates the danger of aspiration. Close patient preferably own glucagon injection technique.
Forecast
Light hypoglycemia in trained patients against the background of good disease compensation is safe. Frequent hypoglycemia are a sign of poor SD compensation; In most cases, such patients during the rest of the day the day is determined by more or less pronounced hyperglycemia and high level of glycated hemoglobin. Elderly patients with late complications of SD hypoglycemia can provoke such vascular complications as myocardial infarction, stroke, retina hemorrhage. The hypoglycemic coma last to 30 minutes with adequate treatment and the rapid return of consciousness, as a rule, does not have any complications and consequences.
7.8. Late complications of diabetes
Late complications are developing with both types of SD. Clinically allocate five main late complications of SD: macroangiopathy, nephropathy, retinopathy, neuropathy and diabetic foot syndrome. The nonspecificity of the late complications for individual types of SD is determined by the fact that their main pathogenetic link is chronic hyperglycemia. In this regard, at the time of the manifestation of SD-1, late complications in patients almost never meet, developing through years and decades, depending on the effectiveness of the therapy. The greatest clinical value at SD-1, as a rule, acquires diabetic microangiopathy(neuropathy, retinopathy) and neuropathy (diabetic foot syndrome). With SD-2, on the contrary, late complications are often detected at the time of the diagnosis. First, this is due to the fact that SD-2 manifests long before the diagnosis is established. Secondly, atherosclerosis, clinically manifested macroangiopathy, has a lot of pathogenesis common with SD. With SD-2, the largest clinical significance, as a rule, acquires diabetic macroangiopathy,which at the time of the diagnosis is detected from the overwhelming majority of patients. In each particular case, the set and severity of individual late complications vary from their paradoxical complete absence, despite the significant duration of the disease up to a combination of all possible options in heavy form.
Late complications are the main cause of deathpatients with SD, and taking into account his prevalence - the most important medical and social problem of health care of most countries. Concerning the main purpose of treatmentand the observations of patients with CD is prevention (primary, secondary, tertiary) of its late complications.
7.8.1. Diabetic macroangiopathy
Diabetic macroangiopathy- a collective concept that combines atherosclerotic lesion of large arteries at SD,
clinically manifested by ischemic heart disease (IBS), oblique atherosclerosis of brain vessels, lower extremities, internal organs and arterial hypertension (Table 7.16).
Table. 7.16.Diabetic macroangiopathy
 Etiology and pathogenesis
Etiology and pathogenesis
Probably similar to the etiology and the pathogenesis of atherosclerosis in individuals without SD. Atherosclerotic plaques do not differ in the microscopic structure in individuals from the SD and without it. Nevertheless, with the SD to the foreground, additional risk factors can be performed, or the SD exacerbates well-known non-specific factors. As such, the SD should include:
1. Hyperglycemia.It is a risk factor for atherosclerosis. An increase in the HBA1C level by 1% in patients with SD-2 increase
the risk of developing myocardial infarction by 15%. The atherogenic action mechanism of hyperglycemia is not quite clear, it is possible that it is associated with the glycosing of the final products of the metabolism of the LDL and the collagen of the vascular wall.
2. Arterial hypertension(AG). In the pathogenesis, great importance is attached to the kidney component (diabetic nephropathy).AG with SD-2 - no less significant infarction and stroke risk factor than hyperglycemia.
3. Dislipidemia.Hyperinsulamia, which is an inalienable component of insulin resistance at SD-2, causes a decrease in the level of HDL, increasing the level of triglycerides and a decrease in density, i.e. Strengthening atherogenic LDL.
4. Obesity,with which most patients with SD-2 suffer, is an independent risk factor atherosclerosis, myocardial infarction and stroke (see paragraph 11.2).
5. Insulin resistance.Hyperinsulamia and high levels of insulin-proinsulin-like molecules increases the risk of atherosclerosis, which may be associated with endothelial dysfunction.
6. Blood coagulation disorder.At the SD, the increase in the level of fibrinogen, the activator of the platelet inhibitor and the Willebrand factor, as a result of which the prothrombotic state of the coagulated blood system is formed.
7. Endothelial dysfunction,characterized by an increase in the expression of the activator of the plasminogen inhibitor and cell adhesion molecules.
8. Oxidative stressleading to an increase in the concentration of oxidized LDL and F2-iso-perts.
9. Systemic inflammationin which the expression of fibrinogen and C-reactive protein increases.
The most significant factors for the risk of developing IWC at SD-2 are an elevated level of LDL, reduced HDL, arterial hypertension, hyperglycemia and smoking. One of the differences of the atherosclerotic process at the SD is more common and the distal nature of the occlusal lesion,those. The process is more often involved in relatively smaller arteries, which makes it difficult to surgical treatment and worsens the forecast.
Epidemiology
The risk of developing the IBS in persons with SD-2 6 times higher than those without diabetes, while it is the same for men and women. Arterial hypertension is detected in 20% of patients with SD-1 and 75% from SD-2. In general, in patients with SD it meets 2 times more often than those without him. Obricultural atherosclerosis of peripheral vessels is developing in 10% of patients with SD. The thromboembolism of the brain vessels develops in 8% of patients with SD (2-4 times more often than those without SD).
Clinical manifestations
Mostly do not differ from those of those without SD. In the clinical picture of the SD-2 macrovascular complications (myocardial infarction, stroke, occlusive damage to the foot vessels) often play the fore, and it is precisely for the first time a hyperglycemia in their development in a patient. Perhaps due to the accompanying autonomous neuropathy to 30% of myocardial infarction in persons with CDs flow without a typical angiosky attack (Bureauless infarction).
Diagnostics
The principles of diagnosis of complications of atherosclerosis (IBS, brainwater impairment, occlusive damage to the arteries of the legs) are not different from those for individuals without SD. Measure arterial pressure(AD) should be carried out on each visit to the patient with the SD to the doctor, and the definition of indicators lipid spectrumblood (total cholesterol, triglycerides, LDL, HDL) at the SD must be carried out at least once a year.
Differential diagnosis
Other cardiovascular diseases, symptomatic arterial hypertension, secondary dlypidemia.
Treatment
♦ Control of blood pressure.The proper level of systolic blood pressure at the SD is less than 130 mmhg, and the diastolic 80 MMNG (Table 7.3). Most patients need several hypotensive drugs to achieve this goal. The drugs for the selection of hypotensive therapy at CD are ACE inhibitors and angiotensin receptor blockers, which, if necessary, are complemented by thiazide diuretics. Selection preparations for patients with SDs that have undergone myocardial infarction are β-adrenoblays.
♦ Dislipidemia correction.Target levels of lipid spectrum indicators are presented in Table. 7.3. Preparations of the selection of hypolipidemic therapy are inhibitors of 3-hydroxy-3-methylglu-co-reductase (statins).
♦ Antiagregant therapy.Aspirin therapy (75-100 mg / day) is shown to patients with sd older than 40 years old with an increased risk of developing cardiovascular pathology (burdened family history, arterial hypertension, smoking, dyslipidemia, microalbuminuria), as well as to all patients with clinical manifestations of atherosclerosis as Secondary prophylaxis.
♦ Screening and treatment of IBS.Load tests for the exclusion of IBS are shown to patients with symptoms of cardiovascular diseases, as well as when identifying pathology at ECG.
Forecast
75% of patients with SD-2 and 35% of patients with SD-1 die from cardiovascular diseases. Approximately 50% of patients with SD-2 die from CHD complications, 15% of brain vessel thromboembolism. Mortality from myocardial infarction in persons with SD exceeds 50%.
7.8.2. Diabetic Retinopathy
Diabetic Retinopathy(DR) - microangiopathy of the retinal vessels of the eye, characterized by the development of microenvironment, hemorrhages, exudative changes and proliferation of newly formed vessels, leading to partial or complete loss of vision (Table 7.17).
Etiology
The main etiological factor in the development of DR is chronic hyperglycemia. Other factors (arterial hypertension, dyslipidemia, smoking, pregnancy, etc.) have less importance.
Pathogenesis
The main links of the pathogenesis of DR are:
Network vessel microangiopathy, leading to a narrowing of the surveillance of vessels with the development of hypoperfusion;
Degeneration of vessels with the formation of micronevity;
Progressive hypoxia, stimulating vessel proliferation and leading to fatty dystrophy and deposition of calcium salts in the retina;
Table. 7.17.Diabetic Retinopathy
 microindarcts with exudation, leading to the formation of soft "cotton spots";
microindarcts with exudation, leading to the formation of soft "cotton spots";
The deposition of lipids with the formation of dense exudates;
The growth in the retina of the proliferating vessels to form shunts and aneurysm, leading to the dilatation of veins and exacerbation of retinal hypoperfusion;
The phenomenon of the trusting with further progression of ischemic, which is the cause of the formation of infiltrates and scars;
Retinal detachment as a result of its ischemic disintegration and formation of vitreoretinal tracts;
Hemorrhages in the vitreous body as a result of hemorrhagic heart attacks, massive vascular invasion and rosing aneurysm;
Proliferation of the vessels of the iris (diabetic rubles), leading to the development of secondary glaucoma;
Maculopathy with swelling swelling.
Epidemiology
Other is the most common cause of blindness among the working population of developed countries, and the risk of development of blindness in patients with diabetes 10-20 times higher than in the overall population. At the time of the diagnosis of SD-1, DR is not detected by almost any of the patients, after 5 years, the disease is detected in 8% of patients, and with a thirty-year period of diabetes - in 98% of patients. At the time of diagnostics, SD-2 dr is detected in 20-40% of patients, and among patients with fifteen years of experience, SD-2 - in 85%. At SD-1, proliferative retinopathy is concerned relatively more often, and at SD-2 - Maculopathy (75% of cases of maculopathy).
Clinical manifestations
According to the generally accepted classification, 3 stages of DR are distinguished
(Table 7.18).
Diagnostics
Complete ophthalmologic examination, which includes direct ophthalmoscopy with the photographing of the retina, is shown to patients with SD-1 after 3-5 years after the disease manifestation, and patients with SD-2 immediately after it is detected. In the future, such research must be repeated annually.
Table. 7.18.Classification of diabetic retinopathy
 Differential diagnosis
Differential diagnosis
Other eye disease in patients with diabetes.
Treatment
The basic principle of treatment of diabetic retinopathy, as well as other late complications, is the optimal compensation of the SD. The most effective method of treating diabetic retinopathy and prevent blindness is laser photocoagulation.Purpose
 Fig. 7.11.Diabetic Retinopathy:
Fig. 7.11.Diabetic Retinopathy:
a) non-proliferative; b) prepolytematic; c) proliferative
laser photocoagulation is the cessation of the functioning of newly formed vessels, which represent the main threat to the development of such heavy complications, like hemophthalm, retinal traction retinal detachment, iris rubbing and secondary glaucoma.
Forecast
The blindness is recorded in 2% of patients with CD (3-4% of patients with SD-1 and 1.5-2% of patients with SD-2). The approximate frequency of new cases of blindness associated with DR is 3.3 cases per 100,000 population per year. During SD-1, the decrease in HBA1C to 7.0% leads to a decrease in the risk of DR development by 75% and reduce the risk of progression by 60%. At SD-2, the decrease in HBA1C by 1% leads to a decrease in the risk of DR development by 20%.
7.8.3. Diabetic nephropathy
Diabetic nephropathy(DNF) is defined as albuminuria (more than 300 mg of albumin per day or proteinurium more than 0.5 g of protein per day) and / or a decrease in the filtering function of the kidneys in individuals from the CD in the absence of urinary infections, heart failure or other kidney disease. Microalbuminuria is defined as the excretion of albumin 30-300 mg / day or 20-200 μg / min.
Etiology and pathogenesis
The main risk factors of the DNF are the duration of SD, chronic hyperglycemia, arterial hypertension, dyslipidemia, kidney disease from parents. When DNF is primarily affected cluster apparatuskidney.
1. One of the possible mechanisms for which hyperglycemiapromotes the development of the lesion of glomers, is the accumulation of sorbitol due to the activation of the polyol path of the glucose metabolism, as well as a number of finite gyricing products.
2. Hemodynamic violations, namely introlubok arterial hypertension(raising blood pressure inside the kidney gloms) is the most important component of pathogenesis
The cause of intraclude hypertension is a violation of the tone of arteriole: the expansion of the resulting and narrowing of the ending.
Table. 7.19.Diabetic nephropathy
 This, in turn, occurs under the influence of a number of humoral factors, such as angiotensin-2 and endothelin, as well as due to the violation of the electrolyte properties of the basal membrane of the glomeruli. In addition, intracralobic hypertension contributes to systemic hypertension, which is determined by most patients with DNF. Due to intracralobic hypertension, there is damage to basal membranes and filtration pores,
This, in turn, occurs under the influence of a number of humoral factors, such as angiotensin-2 and endothelin, as well as due to the violation of the electrolyte properties of the basal membrane of the glomeruli. In addition, intracralobic hypertension contributes to systemic hypertension, which is determined by most patients with DNF. Due to intracralobic hypertension, there is damage to basal membranes and filtration pores,
through which the tracks begin to penetrate (microalbuminuria),and then significant amounts of albumin (proteinuria).The thickening of basal membranes causes a change in their electrolyte properties, which in itself leads to a larger amount of albumin in ultrafiltrate even in the absence of a resizing of filtration pores.
3. Genetic predisposition.The relatives of patients with a DNF with an increased frequency occurs arterial hypertension. There are data on the connection of the DNF with the Polymorphism of the ACE gene. Microscopically, during the DNF, the thickening of the basal membrane of the glomers, expansion of Mezanygia, as well as fibrous changes that bring and enduring arteriols are revealed. At the final stage, which clinically corresponds to chronic renal failure (CPN), the focal (Kimmelistil-Wilson) is determined, and then diffuse glomerosclerosis.
Epidemiology
Microalbuminuria is determined in 6-60% of patients with SD-1 5-15 years after its manifestation. The DNF is determined by 35% from SD-1, more often in men and people who have developed at the age of 15 years old. At SD-2, DNF develops in 25% of representatives of the European race and in 50% of the Asian race. The overall prevalence of DNF at SD-2 is 4-30%.
Clinical manifestations
A relatively early clinical manifestation, which is indirectly connected with the DNF, is arterial hypertension. Other clinically explicit manifestations belong to the late. These include manifestations of nephrotic syndrome and chronic renal failure.
Diagnostics
Screening to the DNF in Persons with CD implies annual testing on microalbuminuriawith SD-1 5 years after the manifestation of the disease, and at SD-2 immediately after it is detected. In addition, it is necessary at least an annual definition of creatinine levels to calculate speed \u200b\u200bof glomerular filtration (SCF).The SCF can be calculated using various formulas, for example, by Cocroft-Golta formula:
For men: a \u003d 1,23 (NOP of SCF 100 - 150 ml / min) for women: a \u003d 1.05 (the norm of SCF 85 - 130 ml / min)
At the initial stages of the DNF, an increase in SCF can be revealed, which gradually falls as CPN developed. Microalbuminuria begins to be determined 5-15 years after the manifestation of SD-1; With SD-2 in 8-10% of cases, it is found immediately after it is detected, probably due to a long asymptomatic course of the disease until diagnosis. The peak of the development of explicit proteinuria or albuminuria at SD-1 falls between 15 and 20 after its start. Proteinuria testifies O. irreversibilityDNF, which sooner or later lead to CPN. Uremia is on average 7-10 years after the appearance of explicit proteinuria. It should be noted that the SCF does not correlate with proteinuria.
Differential diagnosis
Other causes of proteinuria and renal failure in individuals from the SD. In most cases, the DNF is combined with arterial hypertension, diabetic retinopathy or neuropathy, in the absence of which the differential diagnosis should be particularly thorough. In 10% of cases, with SD-1 and in 30% of cases, during SD-2, proteinuria is not related to the DNF.
Treatment
♦ the main conditions of the primary and secondary prevention
DNF.are compensation of SD and maintaining normal systemic blood pressure. In addition, the primary prevention of DNF implies a decrease in protein food consumption - less than 35% of daily calorage.
♦ At the stages microalbuminuriaand proteinuriapatients are shown the appointment of ACE inhibitors or angiotensin receptor blockers. With concomitant arterial hypertension, they are prescribed in hypotensive doses, if necessary in combination with other hypotensive drugs. In normal arterial pressure, these drugs are prescribed in doses that do not lead to the development of hypotension. Both ACE inhibitors (at SD-1 and SD-2) and angiotensin receptor blockers (at SD-2) contribute to preventing the transition of microalbuminuria to proteinuria. In some cases, against the background of this therapy, in combination with the compensation of diabetes for other parameters, the microalbuminuria is eliminated. In addition, starting from the microalbuminuria stage it is necessary
reducing the consumption of proteins is less than 10% of the daily calorage (or less than 0.8 grams per kg of weight) and salts of less than 3 grams per day.
♦ In the Stage CPNas a rule, the correction of sugar therapy is required. Most patients with SD-2 must be translated into insulin therapy, since TSP cumulation carries the risk of severe hypoglycemia. Most patients with SD-1 have a decrease in insulin need, since the kidney is one of the main places of its metabolism. With raising the level of creatinine serum to 500 μmol / l and more need to raise the question of preparing the patient to extracorporeural (hemodialysis, peritoneal dialysis) or surgical (kidney transplantation) treatment method. The kidney transplantation is shown at creatinine levels up to 600-700 μmol / l and reduce the speed of glomerular filtration less than 25 ml / min, hemodialysis - 1000-1200 μmol / l and less than 10 ml / min, respectively.
Forecast
In 50% of patients with SD-1 and 10% with SD-2, which detects proteinuria, CPN develops over the next 10 years. 15% of all patient deaths with SD-1 under the age of 50 are associated with CPN due to the DNF.
7.8.4. Diabetic neuropathy
Diabetic neuropathy(DN) is a combination of nervous system lesions syndromes, which can be classified depending on the prevalent involvement in the process of its various departments (sensorny, autonomous), as well as the prevalence and severity of the lesion (Table 7.20).
I. Sensomotor neuropathy:
Symmetric;
Focal (mononereropathy) or polyphocal (cranial, proximal motor, mononereroid limbs and torso).
II. Autonomous (vegetative) neuropathy:
Cardiovascular (orthostatic hypotension, cardiac denervation syndrome);
Gastrointestinal (stomach atony, dyskinesia of the biliary tract, diabetic enteropathy);
Urogenital (with impaired bladder functions and sexual function);
Violation in the patient's ability to recognize hypoglycemia;
Violation of the pupil function;
Violation of the functions of the sweat glands (distal anhydrosis, hyperhydrosis when eating).
Table. 7.20.Diabetic neuropathy
 Etiology and pathogenesis
Etiology and pathogenesis
The main cause of the day is hyperglycemia. Several mechanisms of its pathogenesis are assumed:
Activation of the polyol route of glucose metabolism, as a result of which sorbitol, fructose and reduction of mioindose and glutathione occurs in nerve cells. This, in turn, leads to the activation of free radical processes and reducing the level of nitrogen oxide;
Non-enzymatic glycosylation of membrane and cytoplasmic proteins of nerve cells;
Microangiopathy vasa Nervorum,which leads to a slowdown in capillary blood flow and nerves hypoxia.
Epidemiology
Prevalence The bottom with both types of SD is about 30%. With SD-1 5 years later, it starts to be detected from 10% of patients. The frequency of new cases of the bottom at SD-2 is about 6% of patients per year. The most frequent option is the distal symmetrical sensitor day.
Clinical manifestations
Sensomotor Dn.manifested by a complex of motor and sensitive disorders. Frequent symptom of distal form paresthesiawhich manifest themselves with the feeling of "crawling of goosebumps", numbness. Patients often complain about the head of the feet, although they remain warm to the touch, which is a sign that allows to distinguish polyneeropathy from ischemic changes when the feet on the touch are cold. An early manifestation of sensory neuropathy is a violation of vibration sensitivity. Characteristic is the "Restless Foot" syndrome, which is a combination of night paresthesia and high sensitivity. Pain in the legsmore often worried at night, while sometimes the patient can take off the touch of the blanket. In a typical case of pain, as opposed to such with obliterating diseases, arteries can decrease when walking. After years, the pain can be spontaneously terminated due to the death of small nerve fibers responsible for pain sensitivity. Hypoesthesiait is manifested by the loss of sensitivity by the type "stocking" and "gloves". The violation of deep, proprioceptive sensitivity leads to a violation of coordination and the difficulty of movement (sensory ataxia). The patient complains about the "Other feet", the feeling of "standing on the cotton". Violation of trophic innervation leads to degenerative changes in skin, bones and tendons. Violation of pain sensitivity leads to frequent, not a noticeable patient with a stop microtraummamm that are easily infected. Violation of coordination and walk leads to non-physiological redistribution of the load on the joints of the foot. As a result, an anatomical relationship in the legistering apparatus of the legs is disturbed.
The arch of the foot is deformed, swelling, fractures, chronic purulent processes develop (see clause 7.8.5).
Several forms of the autonomous day. Cause cardiovascular form- impaired innervation of the cardiovascular complex and large vessels. The wandering nerve is the longest nerve, in connection with which it is amazed before others. As a result of predominance of sympathetic influences develops tahcardia rest.Inadequate response to orthostasis manifests itself ortostatic hypotensionand syncopal states. Vegetative denervation of the pulmonary complex leads to the absence of cardiac rhythm variability. With autonomous neuropathy, the increased prevalence among patients with Miocardial Incecades SD is associated.
Symptoms gastrointestinal shapeThe days are gastroplaz with a slow or, on the contrary, the rapid empty of the stomach, which can create difficulties in the selection of insulin therapy, since the time and volume of suction of carbohydrates vaguely vary; Athony of esophagus, reflux-esophagitis, dysphagia; Water diarrhea. For urogenital formThe bottom is characterized by atony of the ureters and bladder, leading to the inclination to urinary infections; Erectile dysfunction (about 50% of patients with SD); Retrograde ejaculation.
Other possible manifestations of the vegetative day - a violation of the ability to recognize hypoglycemia, violation of the pupil function, violation of the functions of the sweat glands (anhydrosis), diabetic amyotrophyry.
Diagnostics
Neurological examination of patients with SD must be carried out annually. At a minimum, it implies tests aimed at identifying distal sensorin neuropathy. For this, the estimate of the vibration sensitivity is used using graded chateneton, tactile sensitivity with monofilament, as well as temperature and pain sensitivity. According to the testimony, the state of the vegetative nervous system is studied: for diagnosing the insufficiency of parasympathetic heart innervation, a number of functional samples are used, such as measuring the heart rate with deep breathing with evaluation of variability
cardiac rhythm and sample waltasalva; An ortostatic sample is used to diagnose the insufficiency of sympathetic innervation of the heart.
Differential diagnosis
Neuropathy of other genesis (alcoholic, uremic, with 12-identical anemia, etc.). The diagnosis of dysfunction of one or another organ as a result of vegetative neuropathy is established only after the exclusion of organic pathology.
Treatment
1. Optimization of sugar therapy.
2. Care for legs (see clause 7.8.5).
3. The effectiveness of neurotropic drugs (α-lipoic acid) is not confirmed in all studies.
4. Symptomatic therapy (anesthesia, siltenafil with erectile dysfunction, floccortisis with orthostatic hypotension, etc.).
Forecast
In the initial stages, the day can be reversible against the background of the rack compensation of the SD. The bottom is determined in 80% of patients with ulcerative lesions and is the main risk factor amputation
7.8.5. Diabetic foot syndrome
Diabetic foot syndrome(SDS) - the pathological state of the foot at a SD arising against the background of the damage to the peripheral nerves, skin and soft tissues, bones and joints and manifest itself with acute and chronic ulcers, bone-joint lesions and purulent processes (Table 7.21).
Etiology and pathogenesis
The pathogenesis of the SDS is multicomponent, and is represented by a combination of neuropathic and perfusion disorders with a pronounced tendency to infection. Based on the predominance of the pathogenesis of one or another of the listed factors, 3 main forms
Table. 7.21.Diabetic foot syndrome
 I. Neuropathic form(60-70 %):
I. Neuropathic form(60-70 %):
Without osteoarthropathy;
With diabetic osteoarthropathy.
II. Neuriecemic (mixed) form(15-20 %).
III. Ischemic form(3-7 %).
Neuropathic shape of the SDS. In diabetic neuropathy, the distal departments of the longest nerves are affected primarily. A long deficit of trophic impulsation leads to hypotrophy of the skin, bones, ligaments, tendons and muscles. The result of hypotrophy of the connecting structures is the deformation of the foot with non-physiological redistribution of the reference load and its excessive increase in separate areas. In these places, for example in the area of \u200b\u200bprojection heads of tie bones, skin thickening and the formation of hyperkeratosis are noted. The constant pressure on these areas leads to inflammatory autolysis of the toed soft tissues, which creates prerequisites for the formation of a ulcerative defect. As a result of atrophy and disturbance, the skin becomes dry, it is easy to crack. Due to the reduction of pain sensitivity, the patient often does not pay attention to the changes occurring. It cannot timely detect the inconvenience of shoes, which leads to the formation of scuffs and corns, does not notice the introduction of foreign bodies, small wounds in places of cracking. The situation aggravates the impairment of deep sensitivity, manifested in violation of the gait, improper footage. The most often ulcerative defect is infected with staphylococci, streptococci, intestinal group bacteria; Often joins anaerobic flora. Neuropathic osteoarthropathy is the result of severe dystrophic changes in the bonestossert of the foot (osteoporosis, osteolysis, hyperostosis).
Ischemic SDS form it is a consequence of atherosclerosis of the arteries of the lower extremities, leading to a violation of the main blood flow, i.e. It is one of the variants of diabetic macroangiopathy.
Epidemiology
SDS is observed in 10-25%, and according to some data, in one form or another, in 30-80% of patients with diabetes. In the US, the annual spending on the treatment of patients with SD C CDS are 1 billion dollars.
Clinical manifestations
For neuropathic formSDS allocate two most frequent types of lesions: neuropathic ulcer and osteoarthropathy (with development
 Fig. 7.12.Neuropathic ulcer with diabetic foot syndrome
Fig. 7.12.Neuropathic ulcer with diabetic foot syndrome
 Fig. 7.13.Warcot joint with diabetic foot syndrome
Fig. 7.13.Warcot joint with diabetic foot syndrome
charco joint). Neuropathic ulcers,typically, localized in the region of the sole and interpal intervals, i.e. In the sections of the foot experiencing the greatest pressure (Fig. 7.12).
Destructive changes of the binder foot apparatus can progress over many months and lead to severe bone deformation - diabetic osteo arthropathyand formation charcot's jointat the same time, the foot is formally compared with the "bag with bones"
For ischemic form of SDS
the leather in the feet is cold, pale or cyanotic; Less often has a pinkish-red shade of the expansion of superficial capillaries in response to ischemia. Ulcerative defects arise according to the type of acral necrosis - on the tips of the fingers, the edge surface of the heels (Fig. 7.14).
Pulse on the arteries of the foot, popliteal and femoral arteries is weakened or not palpable.
In typical cases, patients impose complaints to "intersecting chromium". The severity of ischemic lesions of the limb is determined by three main factors: the severity of the stenosis, the development of collateral blood flow, the condition of the coagulation system of the blood.
Diagnostics
Inspection of the feet of the patient of the SD should be made every time during a visit to the doctor, not less than once for half a year. SDS diagnostics includes:
 Fig. 7.14.Acral necrosis with the ischemic form of diabetic foot syndrome
Fig. 7.14.Acral necrosis with the ischemic form of diabetic foot syndrome
Inspection of the legs;
Evaluation of neurological status - various types of sensitivity, tendon reflexes, electromyography;
Assessing the condition of arterial blood flow - angiography, dopplerometry, dopplerography;
X-ray of stop and ankle joints;
Bacteriological examination of the wound discharge.
Differential diagnosis
It is carried out with wound processes in the footsteps of another genesis, as well as other occlusive diseases of the vessels of the lower extremities and the pathology of the foot joints. In addition, the clinical forms of SDS must be differentiated (Table 7.22).
Treatment
Treatment neuropathic-infectedsDS forms include a complex of the following events:
Optimization of CD compensation, as a rule, an increase in the insulin dose, and with SD-2 - transfer to it;
Systemic antibiotic therapy;
Full unloading of the foot (this may cause the healing of the ulcers within a few weeks);
Local processing of wounds with removal of hyperkeratose sections;
Caring for legs, the correct selection and wearing special shoes. Timely conducted conservative therapy allows
avoid operational intervention in 95% of cases.
Table. 7.22.Differential diagnosis of clinical SDS forms
 Treatment ischemicsDS forms includes:
Treatment ischemicsDS forms includes:
Optimization of CD compensation, as a rule, an increase in the insulin dose, and with SD-2 - transfer to it;
In the absence of ulcer-necrotic lesions, ergotherapy (1-2-hour walking per day contributing to the development of collateral blood flow);
Revascularization operations on affected vessels;
Conservative therapy: Anticoagulants, aspirin (up to 100 mg / day), if necessary - fibrinolitics, preparations of prostaglandin E1 and prostacyclin.
In the development of extensive purulent-necrotic lesions, with all VDS variants, an amputation is raised.
Forecast
From 50 to 70% of the total number of agitation of the feet are accounted for by patients with diabetes. Foot amputation in patients with SD are produced 20-40 times more often than those without diabetes.
7.9. Sugar diabetes and pregnancy
Gestational diabetes(GSD) is a violation of glucose tolerance, for the first time revealed during pregnancy (Table 7.23). This definition does not exclude that the pathology of carbohydrate exchange could precede the onset of pregnancy. GHS should be distinguished from situations when a woman with diagnosed diabetes (by virtue of age, more often than SD-1) is a pregnancy.
Etiology and pathogenesis
The GES is similar to those at SD-2. The high level of ovarian and placental steroids, as well as an increase in the formation of cortisol of adrenal cortex leads during pregnancy to the development of physiological insulin resistance. The development of the DVS is associated with the fact that insulin resistance, naturally developing during pregnancy, and, therefore, an increased need for insulin in predisposed persons exceeds the functional ability of the β-cells of the PJZ. After delivery with the return of hormonal and metabolic relations to the initial level, it usually passes.
Table. 7.23.Gestational diabetes
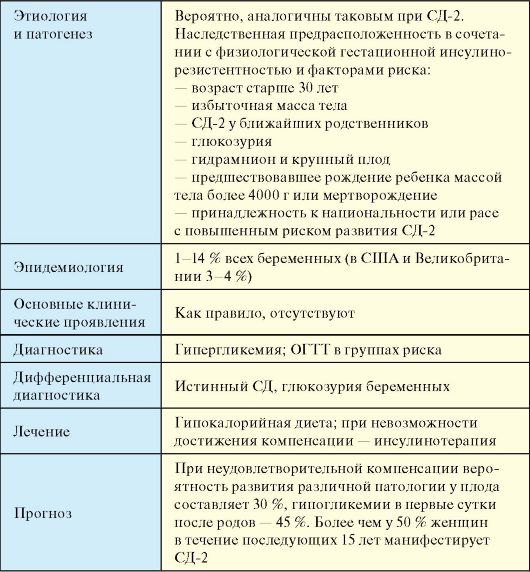 GSD is usually developing in the middle of 2 trimester, between 4 and 8 months of pregnancy. The overwhelming majority of patients have an excess body weight and ae-burdened history. The risk factors of the GDG, as well as groups of women with a low risk of development of the DVS, are shown in Table. 7.24.
GSD is usually developing in the middle of 2 trimester, between 4 and 8 months of pregnancy. The overwhelming majority of patients have an excess body weight and ae-burdened history. The risk factors of the GDG, as well as groups of women with a low risk of development of the DVS, are shown in Table. 7.24.
Table. 7.24.Risk factors for the development of gestational diabetes
 Mother hyperglycemia leads to hyperglycemia in the blood circulation system of the child. Glucose easily penetrates the placenta and continuously moves to the fruit of the mother's blood. The active transport of amino acids and the transfer of ketone bodies of the fruit also occur. In contrast to this insulin, glucagon and the free fatty acids of the mother in the blood of the fetus do not fall. In the first 9-12 weeks of pregnancy, PJZ Fetal has not yet produced its own insulin. This time corresponds to the phase of the fetal organogenesis, when various malformations of development (heart, spine, spinal cord, gastroy) can be formed at the mother's permanent hyperglycemia. Since the 12th week of pregnancy, PJZ Fetal begins to synthesize insulin, and in response to hyperglycemia, reactive hypertrophy and hyperplasia of β-cells of fetal PJZ develop. Due to hyperinsulinmia, the macros of the fetus is developing, as well as the oppression of lecithin synthesis, which explains the high frequency of development of the respiratory distress in newborns. As a result of the hyperplasia of β-cells and hyperinsulinmia, a tendency to heavy and long hypoglycemia appears.
Mother hyperglycemia leads to hyperglycemia in the blood circulation system of the child. Glucose easily penetrates the placenta and continuously moves to the fruit of the mother's blood. The active transport of amino acids and the transfer of ketone bodies of the fruit also occur. In contrast to this insulin, glucagon and the free fatty acids of the mother in the blood of the fetus do not fall. In the first 9-12 weeks of pregnancy, PJZ Fetal has not yet produced its own insulin. This time corresponds to the phase of the fetal organogenesis, when various malformations of development (heart, spine, spinal cord, gastroy) can be formed at the mother's permanent hyperglycemia. Since the 12th week of pregnancy, PJZ Fetal begins to synthesize insulin, and in response to hyperglycemia, reactive hypertrophy and hyperplasia of β-cells of fetal PJZ develop. Due to hyperinsulinmia, the macros of the fetus is developing, as well as the oppression of lecithin synthesis, which explains the high frequency of development of the respiratory distress in newborns. As a result of the hyperplasia of β-cells and hyperinsulinmia, a tendency to heavy and long hypoglycemia appears.
Epidemiology
The CD suffer from 0.3% of all women of reproductive age, 0.2-0.3% of pregnant women are already initially sick, and in 1-14% of pregnancies, the GSD is developing or manifests the true SD. The prevalence of SDS varies in different populations, so, in the US, it is detected in about 4% of pregnant women (135 thousand cases per year).
Clinical manifestations
With the GPS are missing. There may be non-specific symptoms of decompensation of SD.
Diagnostics
Determining the blood glucose level on an empty stomach is shown to all pregnant women within a biochemical blood test. Women who belong to the risk group (Table 7.24), shows oral glucose-beaded test(OGTT). There are many options for its pregnant women. The easiest of them implies the following rules:
3 days before the examination, the woman is on ordinary nutrition and adheres to the usual physical activity;
The test is carried out in the morning on an empty stomach, after night starvation at least 8 hours;
After taking blood sample, an empty stomach woman drinks a solution for 5 minutes, consisting of 75 grams of dry glucose dissolved in 250-300 ml of water; Re-determining the level of glycemia is carried out after 2 hours.
The diagnosis of GSD is set to the following criteria:
Glucose solid blood (venous, capillary) empty stomach\u003e 6.1 mmol / l or
Glucose plasma of venous blood ≥ 7 mmol / l or
Glucose solid capillary blood or plasma of venous blood 2 hours after load 75 g of glucose ≥ 7.8 mmol / l.
If a woman who belongs to the risk group, the results of the study correspond to the norm, the test is reused on the 24-28 week of pregnancy.
Differential diagnosis
GSD and true SD; Glucosuria of pregnant women.
Treatment
The risk for mother and fetus, as well as approaches to the treatment of SD and the features of control over it with the GDG and with the true SD are the same. Late complications of the SD during pregnancy can significantly progress, but with high-quality compensation for SD indications for interrupting pregnancy. A woman suffering from SD (as a rule, we are talking about SD-1), should plan a pregnancy at a young age when the risk of complications of the lowest. If pregnancy is planned, it is recommended to cancel the contact
catching a few months after the achievement of optimal compensation. Contraindications for pregnancy planning are severe nephropathy with progressive renal failure, severe CDS, severe proliferative retinopathy, non-correction, ketoacidosis in early pregnancy (ketone bodies are teratogenic factors).
The purpose of treatmentGSD and true SD during pregnancy is the achievement of the following laboratory indicators:
Spool glycemia< 5-5,8 ммоль/л;
Glycemia 1 hour after meals< 7,8 ммоль/л;
Glycemia 2 hours after meals< 6,7 ммоль/л;
The average value of the daily glycemic profile< 5,5 ммоль/л;
HBA1C level with monthly monitoring, like healthy (4-6%).
With SD-1, as outside of pregnancy, a woman should receive intensive insulin therapy, but the level of glycemia during pregnancy is recommended to evaluate 7-8 times a day. If it is impossible to achieve normoglycemic compensation against the background of ordinary injections, it is necessary to consider the translation of the patient to insulin therapy using insulin dispenser.
At the first stage treatment of GSDdiet therapy is assigned, which consists in limiting the daily calorage of about 25 kcal / kg of actual weight, primarily due to the easiest carbohydrate and animal fats, as well as the expansion of physical exertion. If against the background of diet therapy, it is not possible to achieve the objectives of treatment, the patient must be assigned intensive insulin therapy. Any tableted sugar drugs (TSP) during pregnancy contraindicated.Insulin therapy turns out to be necessary to translate about 15% of women.
Forecast
With unsatisfactory compensation of the DV and SD during pregnancy, the likelihood of various pathology in the fetus is 30% (risk is 12 times higher than in the overall population). More than 50% of women, who during pregnancy, the GDS was detected, for the next 15 years, manifests SD-2.
In the prevention of multifactorial diseases with a hereditary predisposition, to which the EDF is the necessary link ismedical and genetic counseling. The main task of medical and genetic consultation is to determine the genetic risk of the disease and explaining its meaning in an affordable form. When SD into medical and genetic advice, spouses are most often addicted to assess the risk of disease in future children due to the presence of this disease in previous children, or among the spouses themselves and / or their relatives.Population-genetic studies allowed to calculate that the contribution of genetic factors in the development of SD withputs 60-80%. In this regard, the initial relevance and the future acquires the medical and genetic counseling of relatives of patients with SD.
The main issues with which you usually have to face the doctor relate to the risk of developing SD have more children or brothers-sisterspatient, the ability to classify it as well forecast in Titosumming of future (planned) family members.
Consulting families of patients with patients of type 1 type is consisted of several generally accepted stages that have its own characteristics for this contingent.
11.1. Stages of counseling
The first stage of counseling - Summary of the diagnosis of the disease.
Usually the diagnosis of type 1 diabetes in children's and youthful age does not represent difficulties. However, in the presence of SD, other family members need verification of the type of diabetes that in some cases there may be a difficult task and will require a doctor to carefully collect anamnesis of a patient relative. Differential diagnosis between the two main types of SD (1 and 2) is carried out according to generally accepted criteria.
Proven with population-genetic studies genetic heterogeneity of the two main types of SD testifies to their nosological independence and independence of inheritance. This means that those available in the pedigree individual patients with CD 2 types are random and should not be taken into account when evaluating family risk.
When conducting medical and genetic counseling, it is also necessary to exclude genetic syndromes, which include diabetes mellitus, as they are characterized by monogenic inheritance.
The second stage of counseling - determination of the risk of disease development regarding available family members and planned offspring.
Empirical methods were obtained by the average risk assessment of diabetes for members of families with relatives with type 1 SD. The maximum risk has relatives of the I degree of kinship (children, parents, sister brothers) - on average from 2.5-3% to 5-6%. It has been established that the frequency of diabetes disease children from fathers with sd 1 type 1 -2% higherthan from mothers with type 1 type.
In each specific family, the risk of developing the disease depends on many factors: the number of patients and healthy relatives, the age of manifestation of diabetes in family members, the age of consulted, etc.
Table 8.
Empirical risk for relatives of patients SD type 1
On a special technique are calculated risk Tables DevelopmentSD 1. Type depending on the number of patients and healthy relatives and age consulted for families of different types. Families, parent status and the number of patients with Sibs are presented in Table 9.
Down syndrome is not a disease, this pathology is impossible to prevent and cure. The fetus with the Down syndrome in the 21st pair of chromosomes there is a third additional chromosome, as a result of their amount is not 46, but 47. Down syndrome is observed in one of the 600-1000 newborns from women aged after 35. The reason why this happens It is not fully clarified. The doctor from England John Langdon Down first described this syndrome in 1866, and in 1959, French Professor Lezhen proved that this is due to genetic changes.
It is known that half chromosome children get from the mother, and half - from the Father. Since there is not a single effective method of treating Down syndrome, the disease is considered incurable, you can take measures and, if you wish to give birth to a child healthy, contact the medical and genetic advice, where, on the basis of chromosomal analysis, parents will be determined, the child will be born healthy or with Down syndrome.
Recently, such children are born more often, they associate it with late marriage, with pregnancy planning aged 40 years. It is also believed that if the grandmother gave birth to her daughter after 35, then grandchildren can be born with Down syndrome. Although the prenatal diagnosis is a complex survey process, its implementation is very necessary in order to be able to interrupt pregnancy.
What is Down Syndrome. It usually can be accompanied by a delay of motor development. Such children have congenital heart defects, the pathology of the development of the gastrointestinal tract. 8% of patients with Down syndrome are sick leukemia. Medical treatment can stimulate mental activity, normalize hormonal imbalance. With the help of physiotherapy procedures, massages, therapeutic gymnastics, you can help the child to acquire the skills necessary for self-service. Down syndrome is associated with a genetic violation, but it does not always lead to a violation of the physical and mental development of the child. Such children, and in the future adults can participate in all spheres of life, some of them become actors, athletes and can engage in public affairs. How a person will develop with this diagnosis depends largely on the environment in which it grows. Good conditions, love and care contribute to full-fledged development.
Dowon syndrome risk table, by age
The probability of Down syndrome depends on the age of the mother, but it can be revealed by a genetic test in the early stages of pregnancy, and in some cases ultrasound. The likelihood of a child's Down syndrome at birth is lower than at earlier stages of pregnancy, because Some fruits with Down syndrome do not survive.

What risk is considered low, and what is the high?
In Israel, the risk of Down syndrome is considered high, if it is higher than 1: 380 (0.26%). Everyone who is in this risk group needs to be checked by an inflore waters. This risk is equal to risk for those women who pregnant at the age of 35 and older.
The risk is lower than 1: 380 is considered low.
But it must be borne in mind that these borders can be floating! For example, in England, the high level of risk is considered to be higher than 1: 200 (0.5%). This is due to the fact that some women consider the risk of 1 to 1000 - high, and the other 1 to 100 - low, since with such a risk they have a chance for the birth of a healthy child equal to 99%.
Risk Factors Down Syndrome, Edwards, Patau
The main risk factors are age (especially significantly for Down syndrome), as well as the impact of radiation, some heavy metals. It should be borne in mind that even without risk factors, the fruit may have pathology.
As can be seen from the graph, the dependence of the risk of risk from age is the most significant for Down syndrome, and less significant for two other trisomy:

Screening Risk of Down Syndrome
To date, all pregnant women, in addition to relying analyzes, it is recommended to undergo a screening test to identify the degree of risk of Down syndrome by the birth of a child and congenital fetal vices. The most productive survey occurs at 11 weeks + 1 day or at week 13 + 6 days with a coccicco-darkness of the embryo from 45 mm to 84 mm. A pregnant woman can undergo a survey, and use specific ultrasound for this.
A more accurate diagnosis is made using the biopsy of the chorion vane and the study of the amniotic fluid, which is closed using a special needle directly from the fruit bubble. But every woman should know that such methods are conjugate with the risk of pregnancy complications such as miscarriage, fetal infection, the development of hearing loss in the child and much more.
A complete combined screening of the I - II trimester of pregnancy allows you to identify congenital malforms at the fetus. What includes this test? First, an ultrasound study is needed in 10-13 weeks of pregnancy. The calculation of the risk is made to determine the presence of a nasal bone, the width of the cervical fold of the fetus, where the subcutaneous liquid accumulates in the first trimension of pregnancy.
In the seconds, blood test is taken on chorionic gonadotropin in 10-13 weeks and on alpha fetoprotein in 16-18 weeks. The data of combined screening is processed by a special computer program. Scientists proposed a new screening methodology - combining the evaluation of the results obtained during the studies in the first and in the second trimesters. This allows us to ensure a single risk assessment of the occurrence of Down syndrome during pregnancy.
For the first trimester, the results of the determination of RARR-A and measuring the thickness of the collar space are used, and for the second trimester - the combinations of AFP, non-conjugated estriol, xg and inhibin-a are used. The use of an integral assessment for screening survey allows after invasive interventions to reduce the frequency of interrupting the pregnancy for fruits with normal karyotype according to the results of cytogenetic diagnostics.
Integral and biochemical testing for screening Down syndrome allows you to further identify more cases of chromosomal anomalies. This contributes to the prevention of undesirable pregnancy interruptions resulting from amniocentes or biopsy Vorsin Chorion.
Expert editor: Mochalov Pavel Alexandrovich | d. n. therapist
Education: Moscow Medical Institute. I. M. Sechenov, specialty - "Therapeutic Case" in 1991, in 1993 "Professional Diseases", in 1996 "Therapy".
Genetics of diabetes
Prediction of type 1 SD 1 in high-risk groups
T.V.Nikonova, I.I. Grandfather, ji.p. Alekseev, M.N. Boldyreva, O.M. Smirnova, I.V. Dubinkin *.
Endocrinological Scientific Center I (Deer. - Acad. Ramn I. I. Devov) Ramna, I * SSC "Institute of Immunology" I (Deer. - Acad. Ramna P.M. Khaitov) M3 of the Russian Federation, Moscow. I
Currently, there is an increase in the incidence of SD 1 type worldwide. This is due to a number of factors, including an increase in the life expectancy of patients with diabetes due to improving diagnostic and therapeutic assistance, enhanced fertility and deterioration of environmental situations. Reduce the incidence of SD can be carried out by preventive measures, predicting and preventing the development of the disease.
The predisposition to SD 1 type is genetically determined. The incidence of type 1 type 1 is controlled by a number of genes: the insulin gene on chromosome 11p15.5 (Yuom2), genes on chromosome \\\\ c (Yuom4), 6t (Yuom5). The greatest importance from the known genetic markers of type 1 type 1 are genes of the NAA region on chromosome 6p 21.3 (sew1); up to 40% of the genetic predisposition to SD 1 type are associated with them. No other genetic area determines the risk of developing a disease comparable to NA.
The high risk of type 1 type development is determined by allele variants of nuclear genes: December 1 * 03, * 04; OOA1 * 0501, * 0301, OOB1 * 0201, * 0302. 95% of patients with 1 type of SD have an AMA * 3 or 011 * 4 antigens, and from 55 to 60% have both antigen. Allel OOB1 * 0602 is rarely encountered at a type of type 1 and is considered protest.
Clinical manifestations of the diabetes are preceded by a latent period, characterized by the presence of island cellular immunity markers; These markers are associated with progressive destruction.
Thus, for family members with preceding cases of Disease of SD 1 type, disease prediction is particularly important.
The purpose of this work was the formation of high-risk-type development groups in the Russian population of Moscow residents based on the study of genetic, immunological and metabolic markers of diabetes using a family approach.
Materials and research methods
Surveyed 26 families in which one of the parents sick 1 type SD 1, of which 5 are "nuclear" families (only 101 people). The number of surveyed family members ranged from 3 to 10 people. Patients of SD 1 type of fathers - 13, patients with Mat 1 type of mothers are also 13. families in which both parents would be sick 1 type, there was no.
Surveyed 37 descendants of patients with SD 1 type without clinical manifestations of the disease, of which 16 -gen-genus, 21 are male. The age of the examined descendants ranged from 5 to 30 years. The distribution of the examined descendants by age is presented in Table. one.
Table 1
The age of the surveyed children (descendants)
Age (years) number
In families with patients with SD Mothers, 17 children were examined (8 girls, 9 boys), in families with patients with diabetes fathers - 20 children (8 girls, 12 boys).
An autoantile agent (3-cells (ICA) was determined in two ways: 1) on the cryosis of the pancreas of man I (0) of blood groups in the reaction of indirect immunofluorescence; 2) in the ISlettest "ISlettest" firm "Biomerica". An autoantibody for insulin (IAA) was determined in the enzyme-test "islettest" of Biomerica. The definition of antibodies to the DGC was carried out using the standard sets of "Diaplets Anti-Gad" of the company "Boehringer Mannheim".
The definition of the C-peptide was carried out using the standard sets of the company "Sorrin" (France).
The HLA-typing of patients with SD and their families was performed on three genes: DRB1, DQA1 and DQB1 by Sicken-Spec - "" cyphic primers using a polymerase chain reaction (PCR).
The release of DNA from peripheral blood lymphocytes was carried out according to the method R. Higuchi N. Erlich (1989) with some modifications: 0.5 ml of blood taken with EDTA, mixed in 1.5 ml of microcentrifuge tubes such as Eppendorf with 0.5 ml lizuing a solution consisting of 0.32 m sucrose, 10 mM Tris - ns1 pH 7.5, 5mm MgC12, 1% tritone X-100, centrifuged for 1 min at 10,000 rpm, the supernatant was removed, and the sediments of cell cores 2 times washed with the specified buffer. The subsequent proteolysis was carried out in 50 μl of a buffer solution containing 50 mM KCI, 10 mM Tris-ns1 pH 8.3, 2.5 mm Mgci2, 0.45% NP-40, 0.45% twin-20 and 250 μg / ml Proteinases K at 37 "C for 20 minutes. Inactivated proteinase to heating in a solid-state thermostat at 95 "C for 5 minutes. The resulting DNA samples were immediately used to typing or stored at -20 "s. DNA concentration defined by
fluorescence with HOECHST 33258 on the DNA fluorimeter (Hoefer, USA), averaged 50-100 μg / ml. The total time of DNA isolating procedure was 30-40 minutes.
PCR was carried out in 10 μl of the reaction mixture containing 1 μl of DNA sample and the following concentrations of the remaining components: 0.2 mm of each DNTF (DATF, DTTF, DTTF and DGTF), 67mm Tris-HCl pH \u003d 8.8, 2.5 mm Mgc12 , 50 mM NaCl, 0.1 mg / ml gelatin, 1 mm 2-mercaptoethanol, as well as 1 unit thermostable DNA polymerase. To prevent changes in the concentrations of the components of the reaction mixture due to condensate formation, the reaction mixture was covered with 20 μl of mineral oil (Sigma, USA).
Amplification was carried out at the MS2 multichannel thermal cycler (DNA Technology JSC, Moscow).
Typing the DRB1 locus was performed in 2 stages. During the 1st round, the genomic DNA was amplified in two different tubes; In the 1st test tube, a pair of primers amplifying all known alleles of the DRB1 gene, in the 2nd - pair of primers, amplifying only alleles included in the DR3, DR5, DR6, DR8 groups are used. In both cases, the temperature of amplification (for the thermocycler "MS2" with active regulation) was as follows: 1) 94 ° C - 1 min; 2) 94 ° C - 20 C (7 cycles), 67 "C - 2 C; 92 "C - 1 C (28 cycles); 65 ° C - 2 s.
The products obtained were divorced 10 times and used on the 2nd round at the next temperature mode: 92 "C - 1 C (15 cycles); 64 ° C - 1 s.
Typing DQA1 locus was carried out in 2 stages. In the 1st stage, a pair of primers amplifying all the specificity of DQA1 locus was used, on the 2nd - pairs of primers, amplitude-ficizing specificity * 0101, * 0102, * 0103, * 0201, * 0301, * 0401, * 0501, * 0601 .
The first stage was carried out according to the program: 94 "C - 1 min; 94 ° C - 20 C (7 cycles), 58 "C - 5 C; 92" C - 1 C, 5 C (28 cycles), 56 "C - 2 s.
The amplification products of the 1st stage were divorced 10 times and used on the 2nd stage: 93 "C - 1 C (12 cycles), 62" C - 2 s.
Typing the DQB1 locus was also conducted in 2 stages; A pair of primers amplifying all the specificity of the DQB1 locus, the temperature regime is as follows: 94 "C - 1 min.; 94 ° C - 20 s. (7 cycles); 67" C - 5 s.; 93 ° C - 1 C (28 cycles); 65ls - 2 p.
The 2nd stage used pairs of primers, amplifying specificity: * 0201, * 0301, * 0302, * 0303, * 0304, * 0305, * 04, * 0501, * 0502, * 0503, * 0601, * 0602 / 08; The products of the 1st stage were divorced 10 times and amplified in mode: 93 "C - 1 s. (12 cycles); 67" C - 2 s.
Identification of amplification products and their length distribution were performed in ultraviolet light (310 nm) after electrophoresis for 15 minutes or 10% PAAG, 29: 1 at a voltage of 500V, or in 3% agarose gel at a voltage of 300 V (in both cases, mileage was 3-4 cm) and staining with bromide ethidium. As a marker of lengths, the PUC19 plasmid PUC19 Restrictase MSP I was used.
Results and its discussion
It has been established that in 26 families from 26 patients of SD 1 type of parents 23 people (88.5%) are carriers associated with DRB1 * 05-DQA1 * 0501 - DQB1 * 0201 associated with DQB1 * 0201 - DQB1 * 0201; DRB1 * 04-DQAL * 0301-DQB 1 * 0302 or their combinations (Table 2). In 2 patients in the genotype, there is an allele DQB 1 * 0201, associated with a type of type 1; Only 1 patient of this group discovered the DRB1 * 01/01 genotype, which
Distribution of genotypes among patients with sd 1 type of parents
01? In 1 4/4 2 E1? In 1 - - -
Total 23 (88.5%) total 3
0І? B1-ROA-RO Gaplotypes detected by the surveyed persons
ooGvі ooі r)
ry with population studies was not associated with a type of type 1, we did not allocate subtypes of B1 * 04, although the polymorphism of this locus may affect the risk of type 1 SD.
In the genotyping of direct descendants of patients with type 1, it was revealed that out of 37 people 30 (81%) inherited associated with SD 1 of the type of genotypes Ov1 * 03, 011V1 * 04 and their combination, in 3 persons in the genotype there are associated with SD 1 of the allel type 1 : 1 - ooa 1 * 0501, in 2 patients -Oov 1 * 0201. A total of 4 of 37 surveyed are neutral genotype with respect to type 1 type.
The distribution of the genotypes of descendants is indicated in Table. 3. In a number of "works, it is noted that patients of SD 1 type fathers more often transmit genetic predispose
false to diabetes (in particular, NA-01 * 4-genetic types) to their children than mother. However, the study in the UK did not confirm the essential influence of the sex of parents on the nucleus predisposition in children. In our work, we also cannot note such regularity of the transfer of genetic predisposition: from patients with mothers associated with SD 1 type of ny-genotypes inherited 94% of children and from patients with fathers - 85%.
SD, as is known, is a multigenic multifactorial disease. As factors of the external environment playing the role of a trigger, food is considered - breast-consumption and early childhood of proteins of cow's milk. De
Table 3.
The distribution of genotypes among children whose parents are sick 1 type
Gotypes associated with SD 1 type Number of media carriers, not associated with SD 1 type Number of media
0! * In 1 4/4 4 01 * at 1 1/15 1
Total 30 (81%) of only 7 (19%)
with the newly detected diabetes, there are elevated levels of antibodies to a protein of cow milk milk, p-lactoglobulin and bullshit albumin compared to healthy Sibs, which is regarded as an independent risk factor of the SD.
In the group of examined children from 37 people, only 4 were on breastfeeding up to 1 year, 26 people received breast milk to 1.5-3 months, 4 - 6 months, 3 were on dairy blends from the first week of life. Of 5 children with positive antibodies to P-cells 2 were breastfeeding up to 6 months, 3 - to 1.5 - 3 months; Then the kefir and dairy mixtures were obtained. Thus, 89% of the children surveyed received proteins of cow milk at heart age and early childhood, which may be regarded as a risk factor of the SD development in genetically predistened persons.
In the examined families, clinically healthy descendants were determined by cytoplasmic antibodies, autoantibodes to insulin and DGC. Of the 37, the surveyed 5 children were positive on the presence of antibodies to P-cells, while all 5 are carriers of genetic susceptibility to the SD (Table 4). In 3 of them (8%), antibodies to the DGC were found, in 1 - to Azok, in 1 - antibodies to Azok
Table 4.
Gotypes of children positive on antibodies K (3-cells
Gotype number of positive antibodies
and insulin. Thus, antibodies to Azok have 5.4% of children, 2 children with positive antibodies to the DGC are the descendants of the "nuclear" families. The age of children at the time of detection of antibodies is indicated in Table. 5. To predict the SD, the levels of the Azok titer are of great importance: the higher the antibody title, the greater the likelihood of the development of the SD, the same applies to the antibodies to insulin. According to literature, high levels of antibodies to the DGC are associated with a slower pace of development of the SD (10% at 4 years) than low levels (50% at 4 years), perhaps because high levels "of antibodies to the DGC indicate" preferred "Activation of humoral immunity and to a lesser extent to activate cellular
Table 5.
The age of the surveyed children at the time of detection of antibodies
The age of children surveyed (years) the number of children positive on antibodies
immunity (type 1 type SD 1 is mainly due to cell-indirect destruction of p-cells by cytotoxic T-lymphocytes). The combination of various antibodies ensures the most optimal level of forecasting.
In children with low body weight at birth (less than 2.5 kg), diabetes develops significantly earlier than in children born with normal mass. From the data of the anamnesis, the fact that out of 5 children with positive antibodies 2 was born with a mass of the body more than 4 kg, 2-percent of 2.9 kg.
In direct descendants of patients with patients type 1 type, the basal level of the C-peptide has been determined, all this indicator was within the normal range (including in children with positive antibodies to the R-cells), the study of the level of stimulated C-peptide was not conducted.
1. Patients of type 1 type in 88.5% of cases are carriers of the genotypes of derivatives, OOA1 * 0501, VOV1 * 0201, Ov1 * 04, Boa1 * 0301, EOB1 * 0302, or their combinations.
2. In children from families, where one of the parents sick 1 type sample, in 89% of cases, a genetic predisposition to the SD (in the presence of one patient's parent), and 81% inherit fully associated with SD 1 type of genotypes, which allows them to consider them A group of very high risk of diabetes.
3. Among the direct descendants of patients with SD 1 type, having a genetic predisposition, the positive antibodies to the DGC are revealed in 8% of cases, ACOC - in 5.4% of cases. These children need a diagnostic study of antibody titers, glycohemoglobin and the study of insulin secretion.
* 1 iterate
1. ATKINSON M.A., McLaren N.K. // n.Engl.j.med.-L 994 -331. P.L 4281436.
2. Aanstoot H.J., Sigurdsson E., Jaffe M. et al // Diabetologia-1994-37.
3. Baekkeskov S., Aanstoot H.J., Christgan S. et al // Nature-1 990-377.
4. BAIN S.C., ROWE B.R., BARNETT A.H., TODDJ.A. // Diabetes-1994-43 (12). P. 1432-1468.
5. B / NG / EY P.J., Christie M.R., Bonifacio E., Bonfanti R., Shattock MW Fonte M.T., Bottazzo C.f. // Diabetes-1 994-43. P. 1304-1310.
6. Boehn B.O., Manifras B., Seiblerj. et al // Diabetes-1991-40. P.1435-1439.
7. Chern M.M., Anderson V.E., Barbosa J. // Diabetes-1982-31. P.L 1 151118.
8. Davies J.L., Kawaguchi Y., Bennett S.T. et al. // Nature-1994-371.
9. Erlich H.A., Rotter J.I., Chang J. et al. // Nature Gen.-1993-3. P.358-364.
10. Hahl J., Simell T., Ilonen J., Knip M., Simmel O. // Diabetologia-1 99841. P.79-85.
11. Harrison L.C., Honeyman M.C., Deaizpurua H.J. et al // lancet-1993341. P.L 365-1369.
12. HASHIMOTO L., HABITA C., BERESSE J.P. et al. // Nature-1994-371. P.161-164.
1 3. Karjalainen J., Martin J.M., Knip M. et al // n.engl.j.med.-L 992-327. P.302-303.
14. Khan N., Coupert.t., // Diabetes Care-1994-17. P. 653-656.
15. Landin-Oisson M., Palmer J.P., Lernmark A. et al // Diabetologia-1992-40. P.L068-1 073.
16. Leslie R.D.c., ATKINSON M.A., Notkins A.L. // Diabetologia-1999-42. P.3-14.
17. Levy-Marchal C., Dubois F., Neel M., Tichetj., CZERNICHOW P. // Diabetes-1995-44. P.1029-1032.
1 8. Lorenzen T., Pociot F., Hougaard P., Nerup J. // Diabetologia-1994-37. P.321-321.
19. Lorenzen T "Pociot F., Stilgren L. et al // Diabetologia-1998-41. P.666-673.
20. Nepom G., Erlich H.A. // Ann.rev.immunol.-1 991-9. P.493-525.
21. Nerup J., Mandrup-Poulsen T., Molvig J. // Diabetes Metab. Rev.-1987-3. P.779-802.
22. Owerbach D., Gabbay K.H. // Diabetes-1995-44.p.l 32-136.
23. Pociotf. U Dan.med.Bull.-L996-43. P.216-248.
24. Rewers M., Bugawan T.L., Norris J.M., Blair A. et al. // Diabetologia-1996-39. P.807-812.
25. REI / ONEN H., ILONEN J., KNIP N., Akerblom H. // Diabetes-1 991-40.
26. Saukkonen T., Virtanen S.M., Karppinen M. et al // Diabetologia-1998-41. P.72-78.
27. SCHATZ D., Krischerj., Horne G. et al // J. Clin. Invest.-1994-93. P.2403-2407.
28. Spielman R.S., Baker L, Zmijewski C.M. // Ann. Hum. GENET.-1980-44. P. 135-150.
29. Thivolet C., Beaufrere B., Gebuhrer Y., Catelain P., Orgiazzi J. // Diabetologia-1991 -34. P.L86-191.
30. Tillil H., Kobberling J.// Diabetes-1982-36. P.93-99.
31. TODDJ.A. U proc. NATL. ACAD. SCI. USA-1990-377. P.8560-8565.
32. Toddj.a., Farral M. // Hum.mol.gen.-5. P.1443-1448.
33. Tuomilehto J., Zimmet P., Mackay I.R. et al // LanCet-1994-343.
34. Van Der Anvera B., Van WaeyeNegenge C., Schuit F. et al. // Diabetes-1995-44. P.527-530.
35. Walker A. Gudworth AG. // Diabetes-1980-29. P.1036-1039.
36. Warram J., Krolewski A.S., Gottlieb M., Kahn C.R. // n.Engl.j.Med.-1984-311. P.149-151.
37. Ziegler A.G., Herskowitz R.D., Jackson R.A. et al // Diabetes Care-1990-13. P.762-775.




