If you rub the glass wand on a sheet of paper, then the wand will acquire the ability to attract the "Sultan" leaflets, gunki, thin ridges of water. When calculating dry hair, plastic garbage hair is attracted to the calcination. In these simple examples We meet with the manifestation of the forces that were called electrical.
Bodies or particles that act on the surrounding items with electrical forces are called charged or electrified. For example, the glass wand mentioned above after it is lost on a sheet of paper, becomes electrified.
Particles have electric chargeIf they interact with each other through electrical forces. Electrical forces decrease with increasing distance between the particles. Electrical forces are many times higher than the world's strength.
Electric charge is physical quantitywhich determines the intensity of electromagnetic interactions.
Electromagnetic interactions are interactions between charged particles or bodies.
Electrical charges are divided into positive and negative. Stable elementary particles have a positive charge - protons and positrons, as well as the ions of atoms of metals, etc. Stable carriers of the negative charge are an electron and antiproton.
There are electrically uncharged particles, that is, neutral: neutron, neutrino. In electrical interactions, these particles do not participate, since their electrical charge is zero. There are particles without an electric charge, but the electrical charge does not exist without a particle.
On glass, fertilized about Silk, positive charges arise. On the ebony, fell on the fur - negative charges. Particles are repelled during charges of identical signs (charges of the same name), and with different signs (different charges) particles are attracted.
All bodies consist of atoms. Atoms consist of a positively charged atomic nucleus and adversely charged electrons that move around the atomic core. The atomic core consists of positively charged protons and neutral particles - neutrons. The charges in the atom are distributed in such a way that the atom as a whole is neutral, that is, the sum of positive and negative charges in the atom is zero.
Electrons and protons are part of any substance and are the smallest resistant elementary particles. These particles may indeed exist in a free state for a long time. Electric charge of the electron and proton is called an elementary charge.
Elementary charge is a minimum charge that all charged elementary particles have. The proton electric charge is equal than the electron charge:
e \u003d 1,6021892 (46) * 10-19 CL
The magnitude of any charge of a multiple in the absolute value of the elementary charge, that is, the electron charge. The electron is translated from the Greek Electron - Amber, the proton - from the Greek Protos - the first, neutron from the Latin Neutrum is neither one or the other.
Tied with material carrier; The internal characteristic of the elementary particle that determines its electromagnetic interactions.
The electrical charge is a physical quantity characterizing the property of bodies or particles to enter into electromagnetic interactions, and the determining values \u200b\u200bof forces and energies in such interactions. Electrical charge is one of the basic concepts of electricity exercise. All totality electrical phenomena There is a manifestation of existence, movement and interaction of electrical charges. The electrical charge is an integral property of some elementary particles.
There are two types of electrical charges, conventionally called positive and negative. The charges of one sign are repelled, different characters - attract each other. The charge of the electroded glass sticks conditionally began to be considered positive, and the resin (in particular, amber) - negative. In accordance with this condition, the electrical electron charge is negative (Greek. "Electron" is amber).
The charge of the macroscopic body is determined by the total charge of the elementary particles from which this body consists. To charge the macroscopic body, you need to change the number of charged elementary particles contained in it, i.e. transfer to it or remove a certain number of charges of one sign. In real conditions, such a process is usually associated with the movement of electrons. The body is considered charged only if it is an excess of charges of one sign, the body charge, indicated usually by the letter q. or Q. . If the charges are placed on point bodies, the strength of the interaction between them can be determined by the law of the coulon. The charge unit in the SI system is a pendant - CL.
Electric charge q. any body is discretened, there is a minimum, elementary electric charge - e, which is multiple all electrical charges tel:
\\ (q \u003d n e \\)
The minimum charge existing in nature is the charge of elementary particles. In units, the module of this charge is: e. \u003d 1.6.10 -19 CL. Any electrical charges for an integer once more elementary. Elementary electrical charge has all charged elementary particles. At the end of the 19th century An electron-carrier of a negative electric charge was opened, and at the beginning of 20 V, the proton, which is the same largest charge; Thus, it was proved that electric charges exist in themselves, but are associated with particles, are an internal property of particles (other elementary particles carrying a positive or negative charge of the same value were opened). The charge of all elementary particles (if it is not zero) the same in absolute value. Elementary hypothetical particles - quarks whose charge is 2/3 e. or +1/3 e.have not been observed, but in the theory of elementary particles, their existence is supposed.
The invariance of the electrical charge is established experimentally: the value of the charge does not depend on the speed with which it moves (i.e. the value of the charge is invariant with respect to inertial reference systems, and does not depend on whether it moves or rests).
Electric charge additive, i.e. the charge of any system of bodies (particles) is equal to the sum of charge charges (particles) in the system.
The electrical charge is subordinated by the conservation law, which was set after the set of experiments. In an electrically closed system full summary charge It remains and remains constant for any physical processes occurring in the system. This law is fair for isolated electrical closed systems, in which charges are not made and of which they are not taken out. This law acts for elementary particles that are born and annihilated by pairs, the total charge of which is zero.
Simple experiments on the electrification of various bodies illustrate the following provisions.
1. There are charges of two types: positive (+) and negative (-). A positive charge occurs with the friction of glass about the skin or silk, and the negative - with friction of amber (or ebonita) about wool.
2. Charges (or charged bodies) interact with each other. Charged Simony Received, sile, and unlike charges attract.
3. The state of electrification can be transmitted from one body to another, which is associated with the transfer of the electrical charge. At the same time, the body can be transmitted more or less charge, i.e. the charge has a magnitude. With electrification by friction, both bodies acquire, and one is polo-resident, and the other is negative. It should be emphasized that the absolute values \u200b\u200bof charges of electrified friction of bodies are equal, which is confirmed by numerous measurements of the dawn-dov with the help of electrometers.
Explain why the bodies are electrified (i.e. it is charged) by friction, it became possible after opening an electron and studying the structure of the atom. As is known, all substances consist of atoms; Atoms, in turn, consist of elementary particles - adversely charged electronspositively charged protons and neutral particles - neutron. Electrons and protons are carriers of elementary (minimum) electrical charges.
Elementary electric charge ( e.) - This is the smallest electrical charge, put a gear or negative, equal charge value of an electron:
e \u003d. 1,6021892 (46) · 10 -19 CL.
Charged elementary particles there are many, and almost all of them have a charge + E. or -E.However, these particles are very short-lived. They live less than a million dollars of the Cunda. Only electrons and protons exist in a free state for a long time.
Protons and neutrons (nucleons) constitute a positively charged atom core, around which negatively charged electrons rotate, the number of which is equal to the number of protons, so that an atom in general electrocontrauls.
In conventional body conditions consisting of atoms (or molecules), electrically neutral. However, in the process of friction, part of electrons who left their atoms can move from one body to another. The movement of electrons does not exceed the dimensions of the interatomic distances. But if the bodies are disconnected after friction, they will be charged; The body, which gave part of its electrons, will be charged positively, and the body that has acquired them is negative.
So, the bodies are electrified, i.e., they receive an electrical charge when they lose or prior retire electrons. In some cases, electrification is due to the movement of ions. New electric charges do not occur. There is only a separation of existing charge-doves between the electrical bodies: some of the negative charges moves from one body to another.
Definition of charge.
It should be especially emphasized that the charge is an integral part of the particle. It is possible to imagine a particle without charge, but the charge without a particle is impossible.
The charged particles are shown in attraction (different charges) either in repulsion (charges of the same name) with forces, many orders of magnitude exceeding gravitational. Thus, the power of electrical attraction of an electron to the kernel in a hydrogen atom is 10 39 times more of the forces of the gravitational attraction of these particles. Interaction between charged particles is called electromagnetic interaction, and the electrical charge determines the intensity of electromagnetic interactions.
In modern physics, so determine the charge:
Electric charge - this is a physical value that is the source electric fieldBy which the interaction of particles with charge is carried out.
Essay by electrical engineering
Performed: Agafonov Roman
Luzhsky agro-industrial college
It is impossible to give a brief rather satisfactory in all respects. We are accustomed to finding our understanding of the explanations of the very complex formations and processes like an atom, liquid crystals, the distribution of molecules in speeds, etc. But the most basic, fundamental concepts, unnecessary to the simpler, devoid, according to science today, of any internal mechanism, briefly satisfactorily no longer clarify. Especially if objects are not directly perceived by our senses. It is to such fundamental concepts that refers an electric charge.
We will try first to find out nothing that is an electric charge, but what is hidden by the statement of this body or particle have an electric charge.
You know that all the bodies are built from the smallest, indivisible to the simpler (how long is the science) particles that are therefore called elementary. All elementary particles have a mass and thanks to this are attracted to each other. According to the World Act, the attraction force relatively slowly decreases as the distance between them increases: inversely in proportion to the square square. In addition, most elementary particles, although not all, have the ability to interact with each other with force, which also decreases inversely in the square of the distance, but this force is in a huge number, once exceeds the power of gravity. Thus, in the hydrogen atom, schematically depicted in Figure 1, the electron is attracted to the kernel (proton) with force, 1039 times greater than the force of gravitational attraction.
If the particles interact with each other with the forces, which slowly decrease with increasing distance and many times higher than the forces of the global gravity, they say that these particles have an electrical charge. Particles themselves are called charged. There are particles without an electric charge, but there is no electrical charge without a particle.
The interactions between charged particles are called electromagnetic. When we say that electrons and protons are electrically charged, it means that they are capable of interactions of a certain type (electromagnetic), and nothing more. No charge in particles means that it does not detect such interactions. The electrical charge determines the intensity of electromagnetic interactions, just as a mass determines the intensity of gravitational interactions. Electric charge - the second (after mass) is the most important characteristic of elementary particles, which determines their behavior in the environment.
In this way
Electric charge is physical scalar value, characterizing the property of particles or bodies to join electromagnetic power interactions.
Electric charge is denoted by letters Q or Q.
Similarly, the concept of a material point is often used in mechanics, which makes it possible to significantly simplify the solution of many tasks, when studying the interaction of charges, an idea of \u200b\u200ba point charge is effective. The point charge is such a charged body, the dimensions of which are significantly less than the distance from this body to the observation point and other charged bodies. In particular, if they talk about the interaction of two spot chargesMoreover, it is assumed that the distance between the two charged bodies under consideration is much larger than their linear dimensions.
The electrical charge of the elementary particle is not a special "mechanism" in a particle, which could be removed from it, decompose on the components and collect again. The presence of an electrical charge in an electron and other particles means only the existence of certain interactions between them.
In nature there are particles with charges of opposite signs. The proton charge is called positive, and the electron is negative. The positive sign of the charge at the particle does not mean, of course, the presence of special advantages. The introduction of the charges of two characters simply expresses the fact that the charged particles can be drawn and repel. With the same charge signs, the particles are repelled, and with different - attract.
There is no explanation for the existence of two types of electrical charges now. In any case, no fundamental differences between positive and negative charges are detected. If the signs of electrical charges of particles changed to the opposite, the nature of electromagnetic interactions in nature would not change.
Positive and negative charges are very well compensated in the universe. And if the universe is finite, then its full electrical charge is in all likelihood, is zero.
The most remarkable is that the electrical charge of all elementary particles is strictly the same modulo. There is a minimum charge called elementary to which all charged elementary particles have. The charge can be positive as the proton, or negative, like an electron, but the charge module in all cases is the same.
Separate part of the charge, for example, an electron is impossible. This is perhaps the most amazing. No modern theory can explain why the charges of all particles are the same, and not able to calculate the value of the minimum electrical charge. It is determined experimentally with the help of various experiments.
In the 60s, after the number of newly open elementary particles began to grow threateningly, the hypothesis was put forward to the fact that all strongly interacting particles are composite. More fundamental particles were named quarks. The striking turned out to be that quarks should have a fractional electric charge: 1/3 and 2/3 elementary charge. For the construction of protons and neutrons are enough two varieties of quarks. And the maximum number, apparently, does not exceed six.
Create a macroscopic standard of electrical charge units, similar to the length of length - meter, is impossible due to the inevitable charge leakage. Naturally, it would be per unit to accept the electron charge (it is now done in atomic physics). But during the times of Kulon, there was not yet aware of the existence in the nature of the electron. In addition, the electron charge is too small, and therefore it is difficult to use it as a reference.
In the international system of units (s), the charge unit - the pendant is installed using a force force unit:
1 pendant (CL) is a charge passing for 1 s through a cross-section of the conductor at a current in 1 A.
The charge of 1 CL is very large. Two such charges at a distance of 1 km would be pushed away from each other with force, a little less strength with which earth Attracts the load with a mass of 1 tons. Therefore, inform a small body (about several meters size) charge in 1 CL is impossible. Stripping apart from each other, the charged particles would not be able to hold on this body. There are no other forces that would be able to compensate for Coulomb repulsion in nature. But in the conductor, which is generally neutral, to lead the charge in 1 CL is not much labor. Indeed, in a conventional electric light bulb with a power of 100 W at a voltage of 127 V, a current is set, a slightly smaller 1 A. At the same time, a charge, almost equal to 1 CL, is carried out in a cross section.
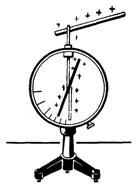
Electrometer is used to detect and measure electrical charges. The electrometer consists of a metal rod and an arrow, which can rotate around the horizontal axis (Fig. 2). The arrow rod is fixed in the plexiglass sleeve and placed in a metal cylindrical body, covered with glass covers.
Principle of operation of the electroometer. Touch a positively charged wand to the electrometer rod. We will see that the electrometer arrow deviates to some angle (see Fig. 2). The turn of the arrow is explained by the fact that when contacting the charged body with the electrometer terminal, electrical charges are distributed throughout the arrow and the rod. The repulsion forces acting between the electrical charges of the electrical charges on the rod and the arrow cause the arrow turn. Electride ebonite wand once again and again touch it the electrometer rod. Experience shows that with an increase in the electrical charge on the rod, the angle of deviation of the arrow from the vertical position increases. Therefore, on the angle of deviations of the arrow of the electrometer, one can judge the value of the electrical charge transmitted by the electrometer rod.
The combination of all known experimental facts allows you to highlight the following charge properties:
There are two kinds of electric charges, conditionally mentioned positive and negative. Positively charged bodies, which act on other charged bodies just like glass, electrified with friction about silk. Negatively charged called the bodies that act just like an ebony, electrified with friction about wool. The choice of the name "Positive" for charges arising on the glass, and the "negative" charges on the ebony are completely accident.
Charges can be transmitted (for example, with direct contact) from one body to another. Unlike body weight, an electrical charge is not an integral characteristic of this body. The same body in different conditions May have a different charge.
The charges of the same name are repelled, the variepetes are attracted. This also shows a fundamental difference. electromagnetic forces From gravitational. Gravitational forces are always attraction forces.
An important feature of the electric charge is its discreteness. This means that there is some smallest, universal, then not a divisible elementary charge, so the charge q of any body is a multiple of this elementary charge:
,where n is an integer, e is the magnitude of the elementary charge. According to modern ideas, this charge is numerically equal to the electron charge E \u003d 1.6 ∙ 10-19 CL. Since the magnitude of the elementary charge is quite small, then for most of the charged bodies observed and used in practice, the number N is very large, and the discrete nature of the charge change is not manifested. Therefore, it is believed that under normal conditions, the electric charge bodies varies almost continuously.
The law of conservation of an electric charge.
Inside a closed system, with any interactions, the algebraic amount of electrical charges remains permanent:
.Insulated (or closed) system we will call a system of bodies, into which electric charges are not introduced and are not displayed.
Nowhere and never occur in nature and does not disappear electrical charge of one sign. The appearance of a positive electric charge is always accompanied by the appearance of a negative charge of an equal chase. Neither a positive nor negative charge can disappear separately, they can only neutralize each other if they are equal to the module.
So elementary particles are able to turn into each other. But always at the birth of charged particles there is a pair of particles with charges of the opposite sign. There may be a simultaneous birth of several such pairs. The charged particles disappear, turning into neutral, too, only in pairs. All these facts leave no doubt in strictly implementing the law of conservation of an electric charge.
The reason for the preservation of the electric charge is still unknown.
Body electrification
Macroscopic bodies are usually electrically neutral. Neutracted atom of any substance, since the number of electrons in it is equal to the number of protons in the nucleus. Positive and negatively charged particles are connected with each other electrical forces and form neutral systems.
The body of large sizes is charged in the case when it contains an excess number of elementary particles with one charge sign. A negative body charge is due to an excess of electrons compared to protons, and a positive charge is their disadvantage.
In order to get an electrically charged macroscopic body or, as they say, to electrify it, it is necessary to separate the part of the negative charge from the associated positive.
The easiest way to do this by friction. If we spend the comb in the hair, then a small part of the most movable charged particles - electrons - will go from the hair on a comb and charges it negatively, and the hair will be charged positively. With electrification by friction, both bodies acquire opposite on the sign, but the same charges are the same.
Electrical body with friction is very simple. But to explain how this happens, it turned out to be a very difficult task.
1 version. When electrification, the bodies are important contact between them. Electrical forces hold electrons inside the body. But for different substances These forces are different. With a close contact, a small part of the electrons of that substance that has the connection of electrons with the body relative to weak, goes to another body. The movement of electrons does not exceed the dimensions of the interatomic distances (10-8 cm). But if the bodies are disconnected, both will be charged. Since the surfaces of the bodies are never perfectly smooth, then the necessary close contact between the bodies is installed only in small areas of surfaces. With friction of bodies, the number of sections with close contact increases, and thereby increases total number charged particles moving from one body to another. But it is not clear how in such non-current substances (insulators), as an ebony, plexiglas and others, electrons can move. They are associated in neutral molecules.
2 version. Using the example of the LIF ion crystal (insulator), this explanation looks like this. When forming a crystal, various kinds of defects arise, in particular, the vacancies - unfilled in nodes crystal lattice. If the number of vacancies for positive lithium and negative ions - fluorine is not the same, then the crystal will be in the formation charged by volume. But the charge as a whole cannot be maintained at the crystal for a long time. There is always some ions in the air, and the crystal will pull them out of the air until the crystal charge is neutralized by the layer of ions on its surface. In different insulators, surround charges are different, and therefore the charges of surface layers of ions are different. When friction, the surface layers of ions are mixed, and when the insulators are disconnected, each of them turns out to be charged.
And can there be two identical insulator with friction with friction, for example, the same LIF crystals? If they have the same own bulk charges, then no. But they can have various own charges if the crystallization conditions were different and a different number of vacancies appeared. As experience showed, electrification by friction of the same crystals of ruby, amber, etc. really can occur. However, the explanation is hardly correct in all cases. If the bodies consist, for example, from molecular crystals, then the appearance of vacancies they should not lead to body charges.
Another method of electrification bodies - the effect on them of various radiation (in particular, ultraviolet, x-ray and γ-radiation). This method is most effective for the electrification of metals, when electrons are erected under the action of radiation from the metal surface, and the conductor acquires a positive charge.
Electrification through influence. The conductor is charged not only when contacting the charged body, but in the case when it is at some distance. Explore Read more this phenomenon. Suspension on an insulated conductor Light paper sheets (Fig. 3). If the conductor is first not charged, the sheets will be in a non-definite position. Approach now to the conductor is an insulated metal ball, strongly charged, for example, with a glass stick. We will see that the sheets suspended in the ends of the body at points A and B are deflected, although the charged body does not concern the conductor. The conductor charged through the influence, why the phenomenon itself received the name "Electrization through influence" or "Electrical induction". Charges received by means electric induction, called induced or induced. Sheets suspended at the middle of the body, at points A 'and B' do not deviate. So induced charges occur only at the ends of the body, and it remains neutral, or uncharged. Running to the sheets suspended at the points A and B, the electrical glass wand, it is easy to make sure that the sheets at the point b are repelled from it, and the sheets at the point are attracted. This means that at the remote end of the conductor there is a charge of the same sign as on the ball, and on the surrounding parts there are charges of another sign. Removing a charged ball, we will see that the sheets will be devastated. The phenomenon proceeds in a completely similar way, if you repeat the experience, charging the ball is negative (for example, with the help of Surguche).
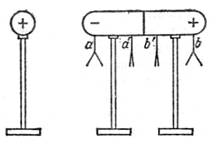
From the point of view of the electronic theory, these phenomena are easily explained by the existence in the conductor of free electrons. When submission to the conductor positive charge Electrons are attracted to it and accumulate at the nearest end of the conductor. It turns out a number of "excess" electrons, and this part of the conductor is charged negatively. At the remote end, the lack of electrons is formed and, consequently, an excess of positive ions: a positive charge appears here.
When submission to the conductor of a negatively charged body, the electrons accumulate at the remote end, and in the near end, an excess of positive ions is obtained. After removing the charge, which causes the movement of electrons, they are again distributed through the conductor, so that all parts of it turn out to be still uncharged.
Moving charges on the conductor and their accumulation at the ends will continue until the impact of excess charges formed at the ends of the conductor does not balance those outgoing from the ball electric powerunder the influence of which the electron is redistributed. The lack of charge in the middle of the body shows that the forces emanating from the ball, and the forces with which they act on the free electrons of excess charges, which have accumulated on the ends of the conductor, are balanced.
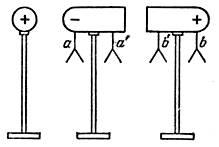
Induced charges can be divided if in the presence of a charged body to split the conductor to pieces. Such experience is depicted in Fig. 4. In this case, the mixed electrons can no longer return after removal of the charged ball; Since there is a dielectric (air) between both parts of the conductor. Excess electrons are distributed over the entire left; The disadvantage of electrons at the point B is partially replenished from the point of the point B ', so each part of the conductor turns out to be charged: the left - charge, the sign of the opposite charge of the ball, the right-charge, the same name with the ball charge. Not only the sheets at points A and B are diverged, but also remaining fixed sheets at the points A 'and B'.
Burov L.I., Strelchyya V.M. Physics from A to Z: Students, Applicants, Tutors. - MN: Paradox, 2000. - 560 p.
Myakyshev G.Ya. Physics: electrodynamics. 10-11 kl.: Studies. For in-depth study Physics /G.I. Myakyshev, A.Z. Synyakov, B.A. Slobodskov. - M.ZH DROF, 2005. - 476 p.
Physics: studies. Manual for 10 cl. shk. and classes with a depth. Research. Physics / O. F. Kabardin, V. A. Orlov, E. E. Eventer, etc.; Ed. A. A. Pinsky. - 2nd ed. - M.: Enlightenment, 1995. - 415 s.
Elementary textbook physics: Tutorial. In 3 tons / ed. G.S. Landsberg: T. 2. Electricity and magnetism. - M: Fizmatlit, 2003. - 480 p.




