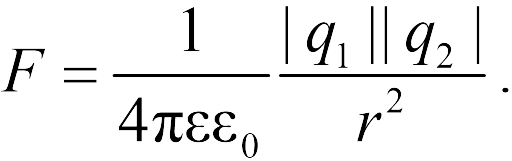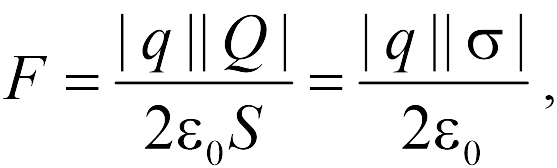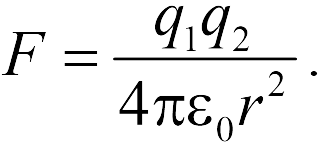The law of Kulon. - This is a law describing the strengths of interaction between point electrical charges.
The module of the interaction force of two point charges in a vacuum is directly proportional to the product of the modules of these charges and inversely proportional to the square of the distance between them.
Otherwise: Two point charges in vacuum They act on each other with the forces that are proportional to the product of the modules of these charges, are inversely proportional to the square of the distance between them and are directed along the straight line connecting these charges. These forces are called electrostatic (Coulomb).
It is important to note that in order for the law to be faithful, it is necessary:
the discretion of charges - that is, the distance between the charged bodies is much larger than their sizes - however, it can be proved that the strength of the interaction of two volumetric charges with spherically symmetric non-volatile spatial distributions is equal to the interaction of two equivalent spot charges placed in spherical symmetry centers;
their immobility. Otherwise, additional effects come into force: a magnetic field The moving charge and the corresponding additional lorentz poweracting on another moving charge;
interaction B. vacuum.
However, with some adjustments, the law is also fair for charge interactions in the environment and for moving charges.
In vector form in the wording of S. Kulon, the law is written as follows:
where - the force with which the charge 1 is valid for charge 2; - the magnitude of charges; - radius vector (vector directed from charge 1 to charge 2, and equal, module, distance between charges -); - proportionality coefficient. Thus, the law indicates that the same charges are repelled (and the relevant - attract).
IN SGSE unit Charge is chosen in such a way that the coefficient k. equal to one.
IN International Unit (SI) One of the main units is one forces electric current ampere, and the unit charge - pendant - derived from him. The amount of ampere is defined in such a way that k. \u003d C 2 · 10 -7 GN/ m \u003d 8,9875517873681764 · 10 9 N.· M 2 / CL 2 (or F -1 · m). In si coefficient k. Recorded in the form:
where ≈ 8,854187817 · 10 -12 f / m - electric constant.
Two point charges act on each other with force, which is inversely proportional to the square of the distance between them and is directly proportional to the work of their charges (excluding the charge sign)
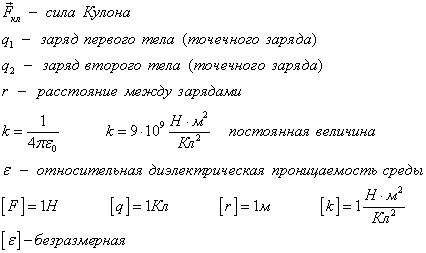
In various environments, such as in the air and in water, two point charges interact with different strengths. The relative dielectric permeability of the medium characterize this difference. This is a well-known table value. For air.
Constant K is defined as
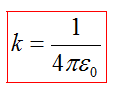
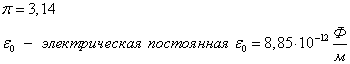
Direction of the power of Coulomb
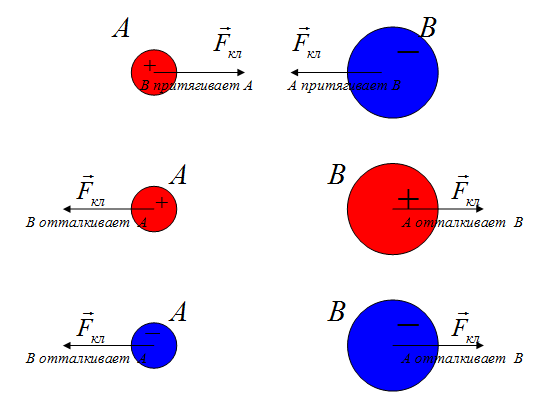
According to the third law of Newton, the forces of one nature arise in pairs, equal in size, opposite to the direction. If two unequal charge interacts, the force with which greater charge It acts on a smaller (in a) is equal to force with which the smaller acts on a larger (and on B).
Interestingly, various physics laws have some common features. Recall the law of gravity. The force of gravity is also inversely proportional to the square of the distance, but already between the masses, and the idea is involuntarily arising that in this pattern a deep meaning is linked. Until now, anyone has managed to present a lot and electricity as two different manifestations of the same entity.
Strength and here it changes in inversely in the square of the distance, but the difference in the magnitude of the electric forces and forces is amazing. Trying to establish the common nature of the electricity and electricity, we discover such a superiority of the electric forces on the forces of grave that it is difficult to believe that those and the other and the same source. How can we say that one acts stronger than the other? After all, it all depends on what the mass and what is the charge. Correcting how much actually acts, you are not entitled to say: "Take a lot of such a value," because you choose it yourself. But if we take what nature itself offers us (its own numbers and measures that have nothing to do with our inches, years, with our measures), then we can compare. We will take an elementary charged particle, such, for example, as an electron. Two elementary particles, two electrons, due to the electrical charge repel each other with force, inversely in a proportional square of the distance between them, and due to the gravity, they are attracted to each other again with force, inversely proportional to the square square.
Question: What is the relationship of strength to electrical power? Communication refers to electrical repulsion, as a unit to a number with 42 zeros. This causes deepest perplexity. Where could this huge number come from?
People are looking for this huge coefficient in other phenomena of nature. They move all sorts of large numbers, and if you need a large number, why not take, say, the ratio of the diameter of the universe to the proton diameter - or surprisingly, this is also a number with 42 zeros. And so they say: maybe this coefficient is equal to the ratio of the proton diameter to the diameter of the universe? This is an interesting thought, but since the Universe gradually expands, constant gravity should change. Although this hypothesis is not yet refuted, we have no evidence in her favor. On the contrary, some evidence suggests that constant gravity has not changed in this way. This is a huge number to this day remains a mystery.
The main law of interaction of electrical charges was found by Charlock Pendant in 1785. Experimentally. Pendant found that the power of the interaction between two small charged metal balls is inversely proportional to the square square 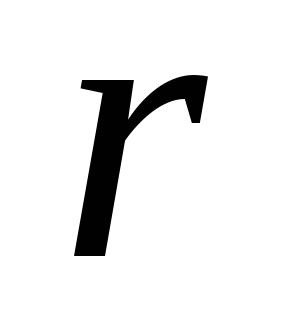 between them and depends on the values \u200b\u200bof charges
between them and depends on the values \u200b\u200bof charges 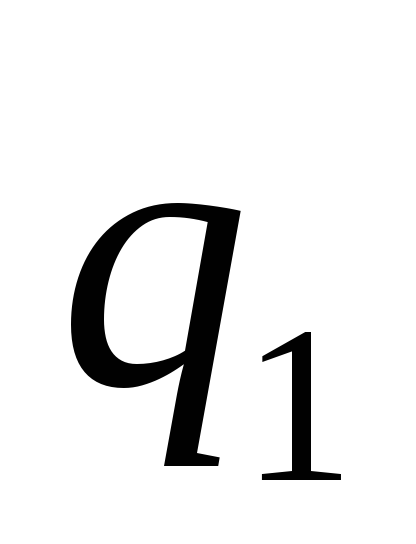 and
and 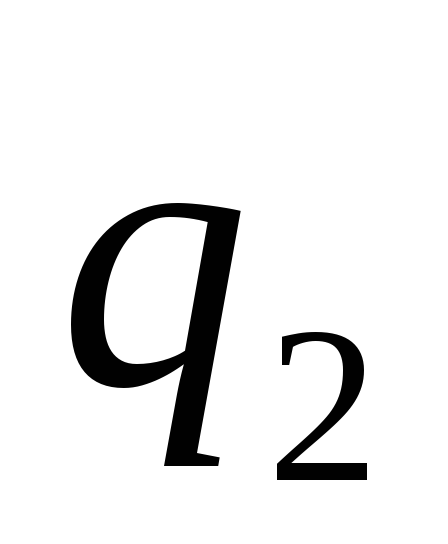 :
:
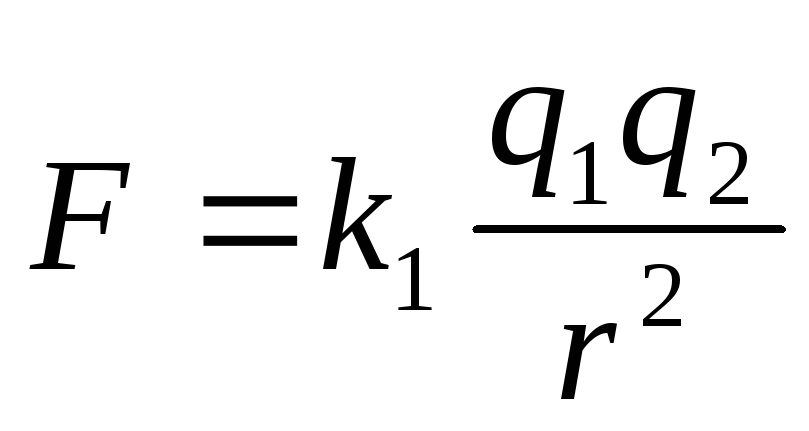 ,
,
where 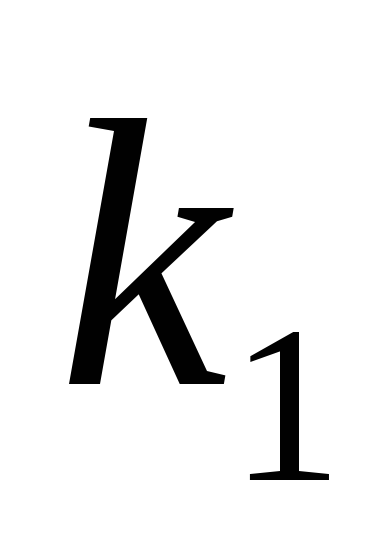 -proportionality coefficient
-proportionality coefficient
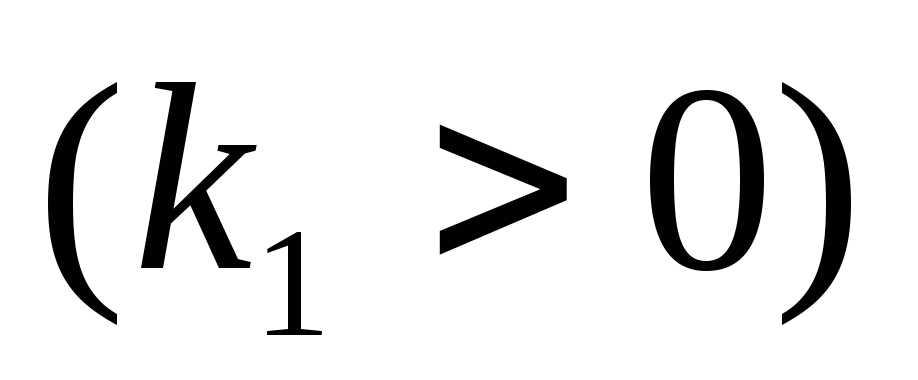 .
.
Forces acting on chargesare central
, that is, they are directed along a straight line connecting charges. 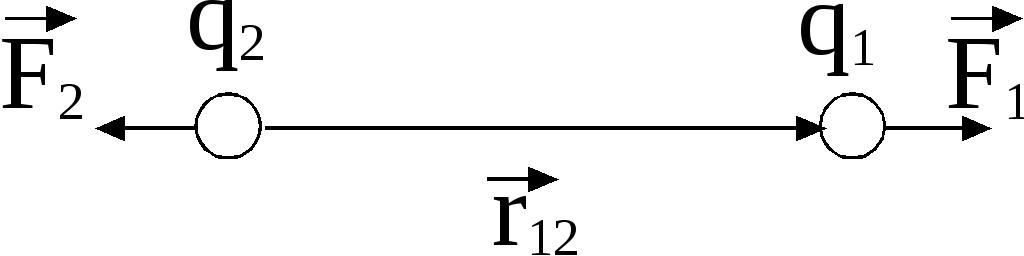
The law of Kulon. can be recorded in vector form: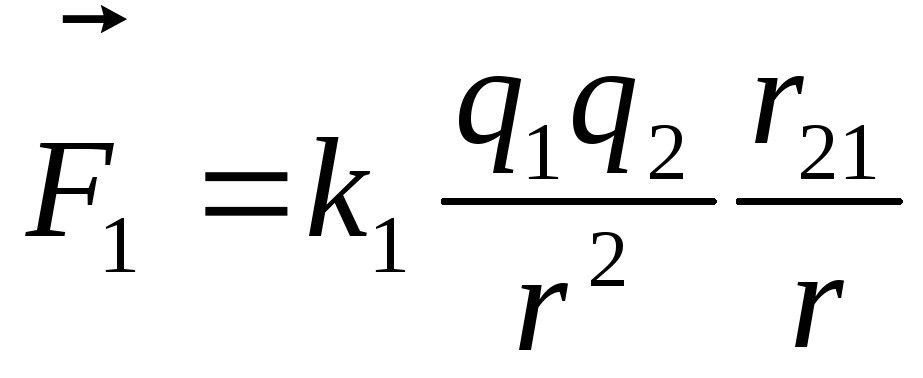 ,
,
where 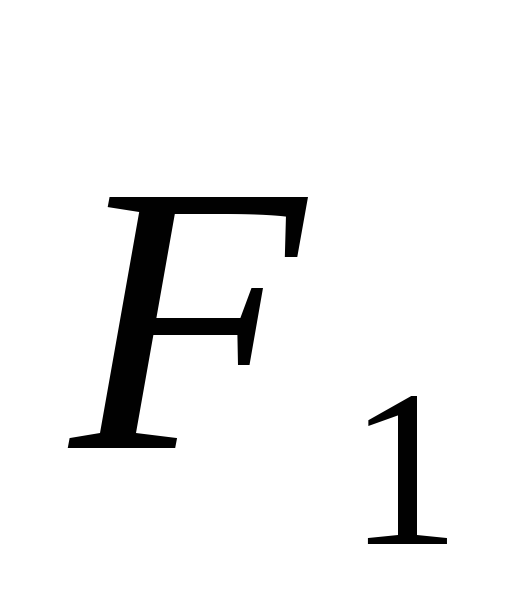 -
-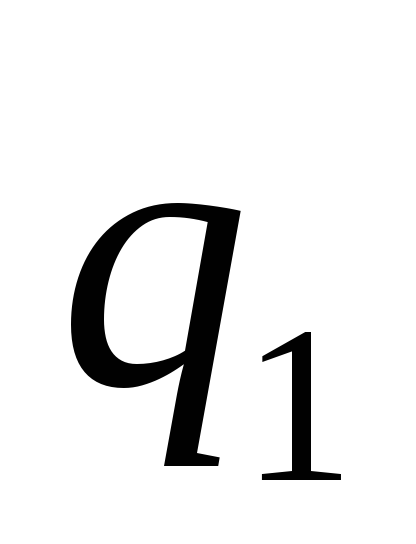 from charge
from charge 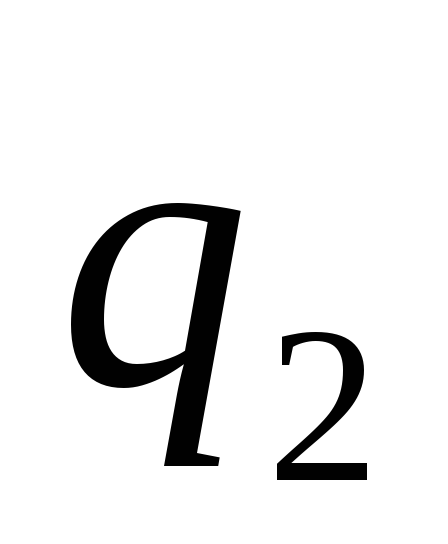 ,
,
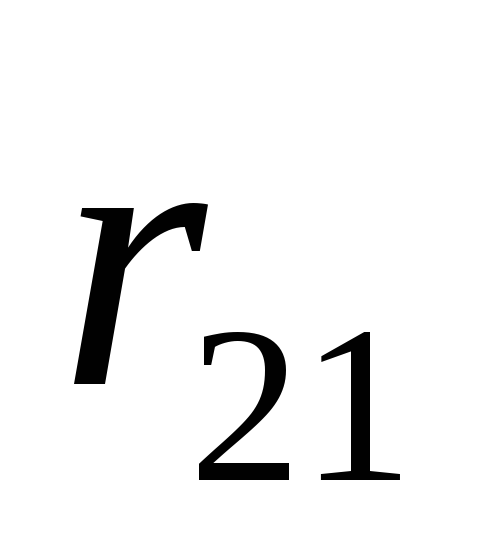 - radius-vector connecting charge
- radius-vector connecting charge 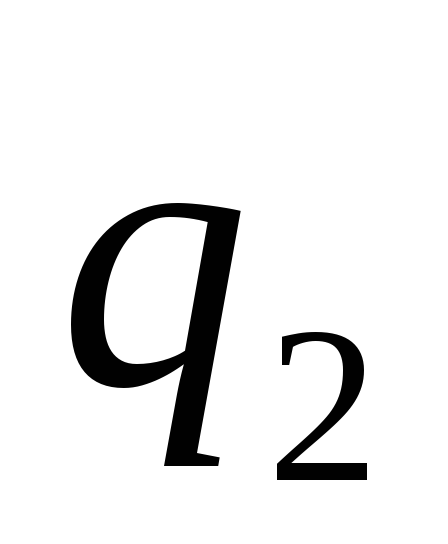 with charge
with charge 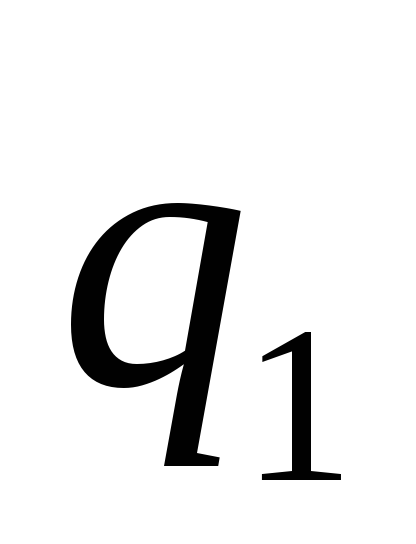 ;
;
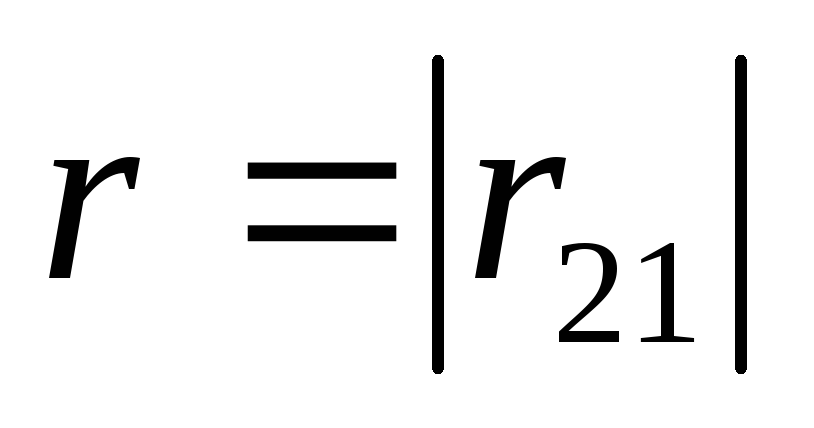 - Module radius-vector.
- Module radius-vector.
Power acting on charge 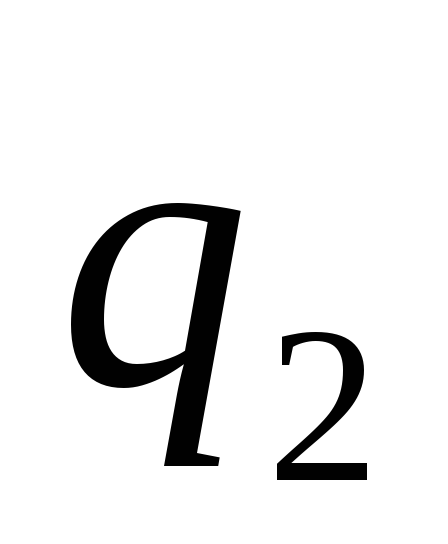 from side
from side 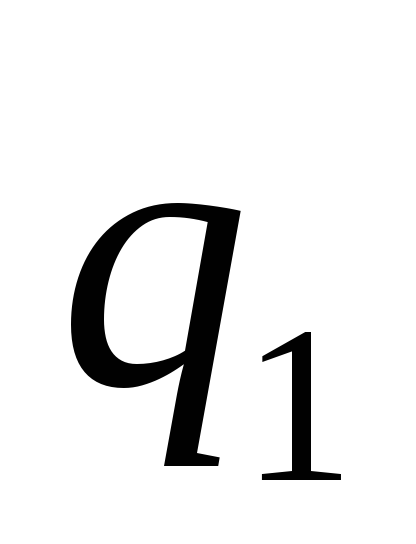 equal
equal 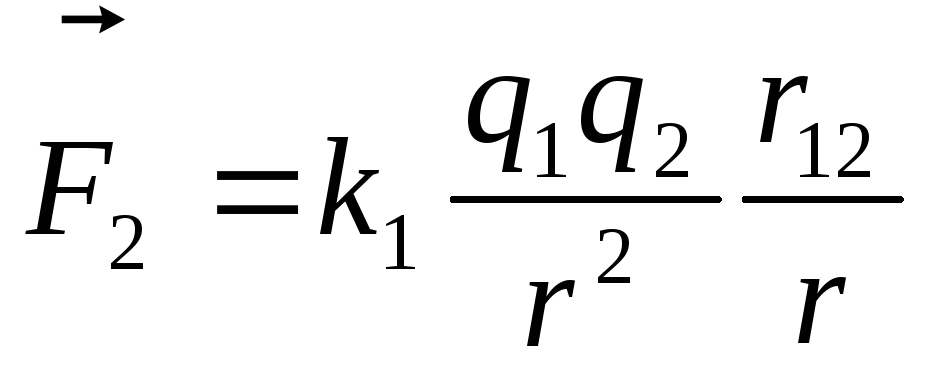 ,
,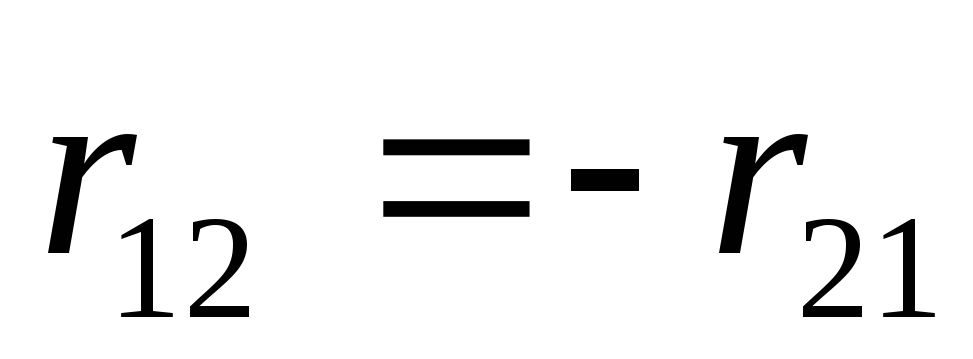 .
.
Cool law in such form
fair only for interaction of point electrical charges, that is, such charged bodies, the linear dimensions of which can be neglected compared with the distance between them.
expresses the strength of interaction Between fixed electrical charges, that is, this is an electrostatic law.
Formulation of the law of Kulon.:
The power of electrostatic interaction between two point electric charges is directly proportional to the product values \u200b\u200bof charges and inversely proportional to the square of the distance between them.
Proportionality coefficient
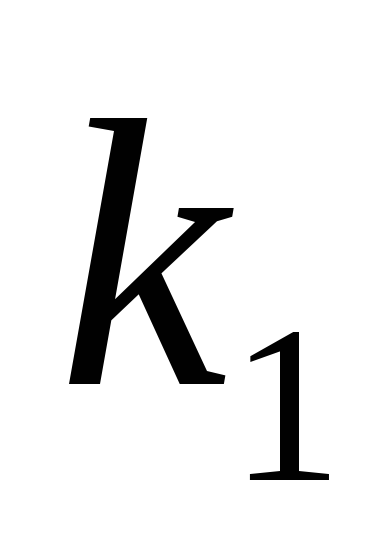 in the law of Kulon depends
in the law of Kulon depends
from the properties of the environment
the choice of units of measurement of the values \u200b\u200bincluded in the formula.
therefore 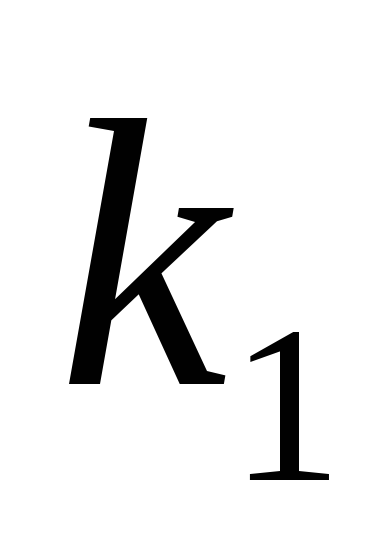 can be represented by attitude
can be represented by attitude 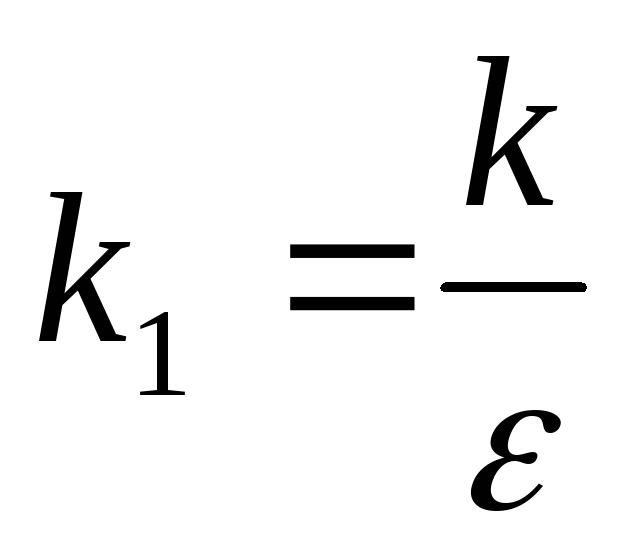 ,
,
where 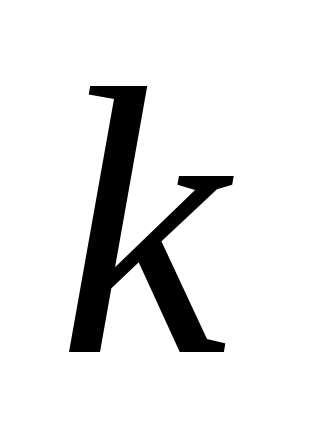 -the coefficient depending only on the choice of system units;
-the coefficient depending only on the choice of system units;
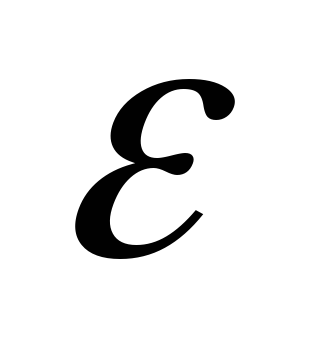 - a dimensionless value that characterizes the electrical properties of the medium is called relative dielectric permeability Environments
. It does not depend on the choice of the system of units of measurement and is equal to one in vacuo.
- a dimensionless value that characterizes the electrical properties of the medium is called relative dielectric permeability Environments
. It does not depend on the choice of the system of units of measurement and is equal to one in vacuo.
Then the law of Kulon will take the form: 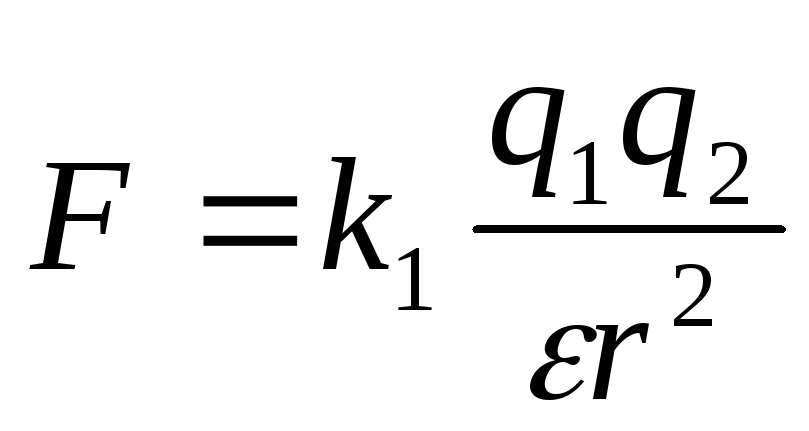 ,
,
for Vacuum 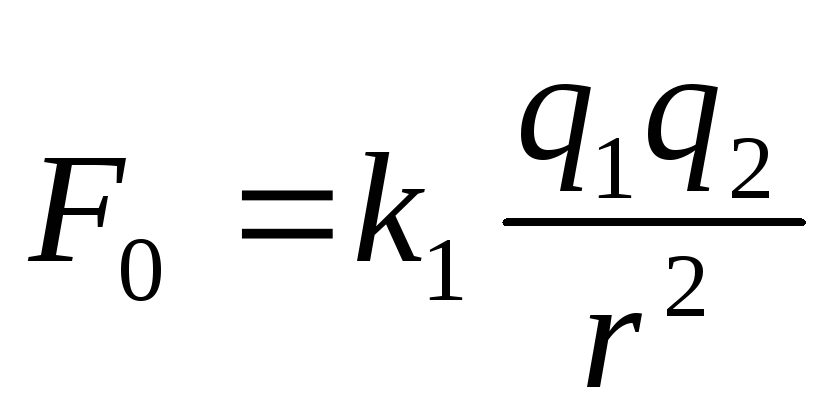 ,
,
then 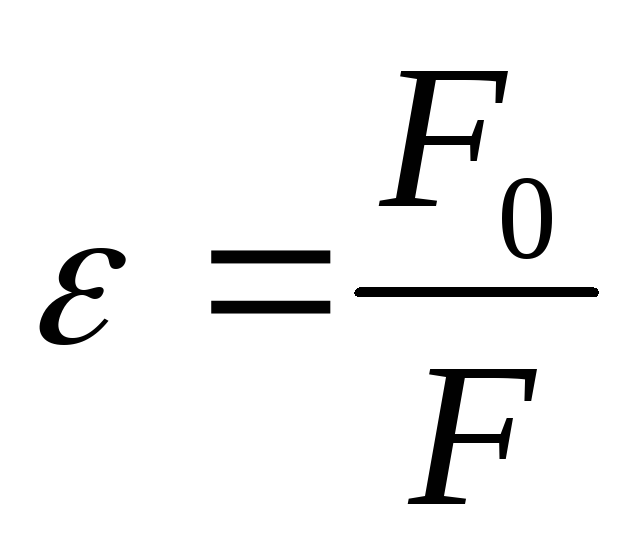 -relative dielectric permeability of the medium shows how many times in this medium the power of the interaction between two point electric charges
-relative dielectric permeability of the medium shows how many times in this medium the power of the interaction between two point electric charges 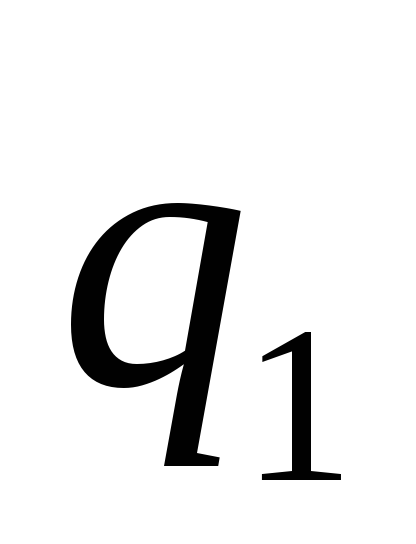 and
and 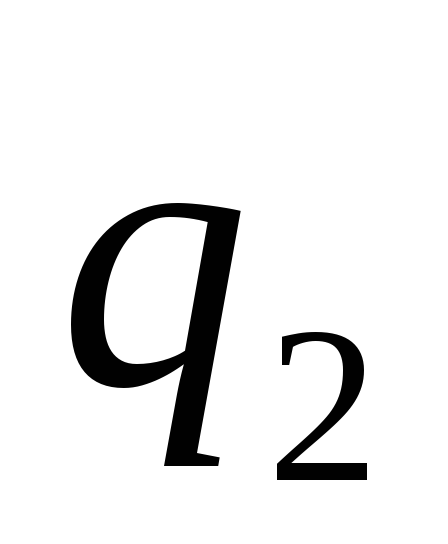 located apart from each other
located apart from each other 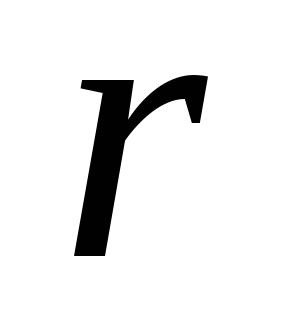 , less than in vacuum.
, less than in vacuum.
In the system S.coefficient 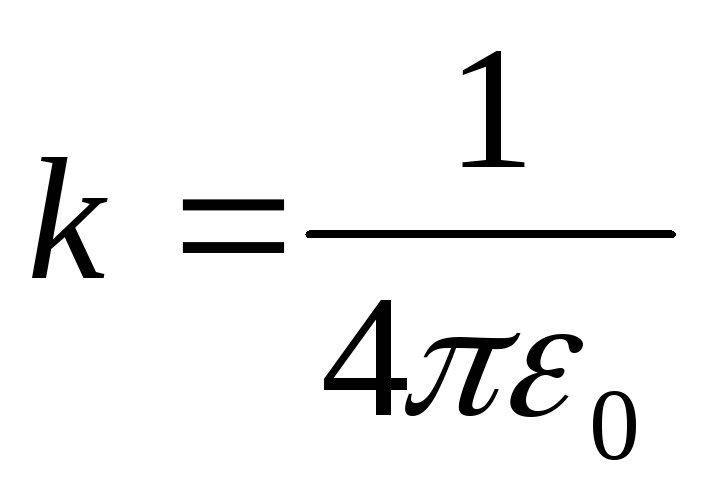 , I.
, I.
the law of Kulon has a view: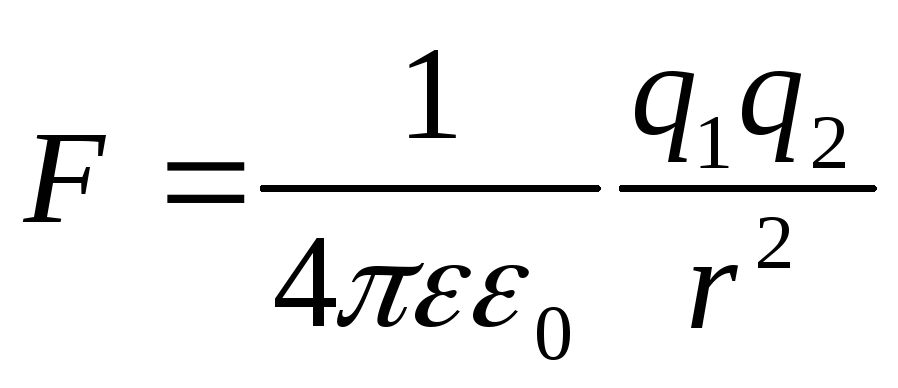 .
.
it rationalized record of the lawulon.
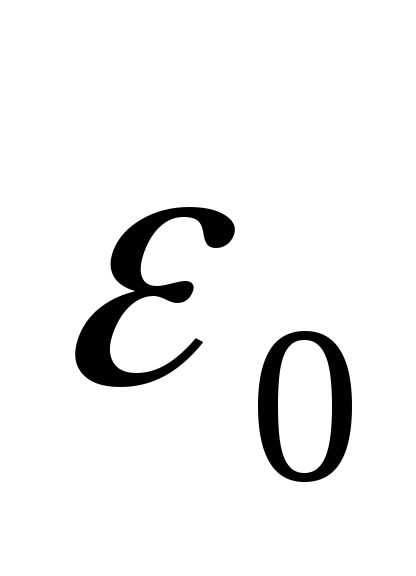 - electric constant,
- electric constant, 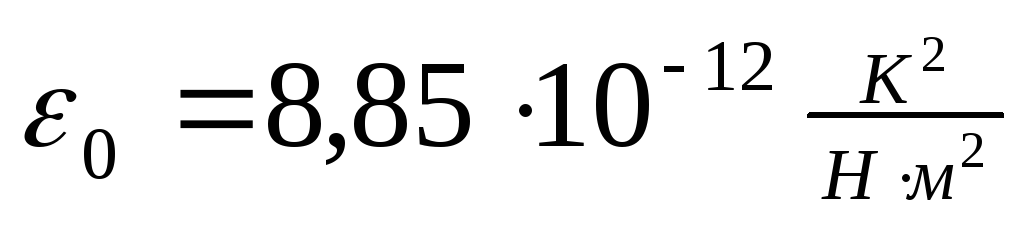 .
.
In the SGSE system
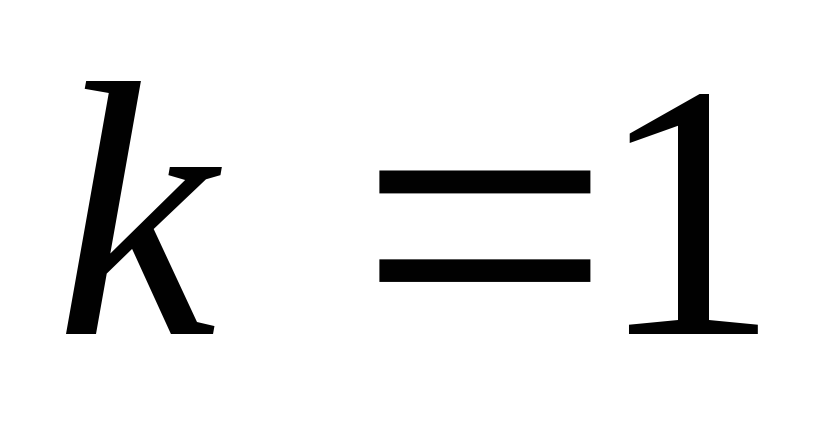 ,
,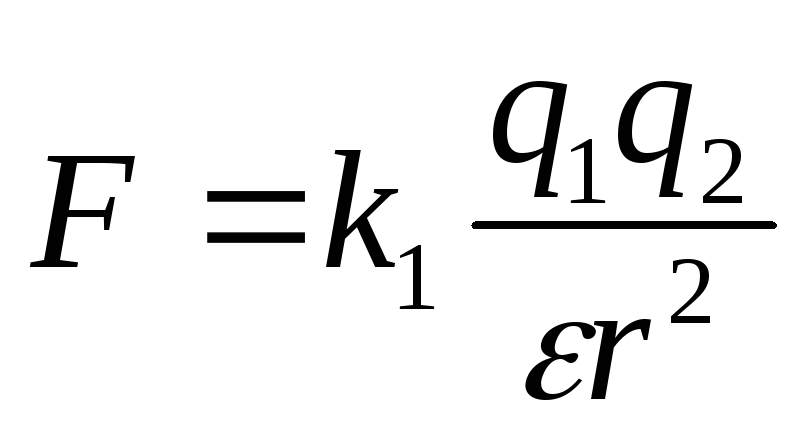 .
.
In vector form the law of the coulon Takes species 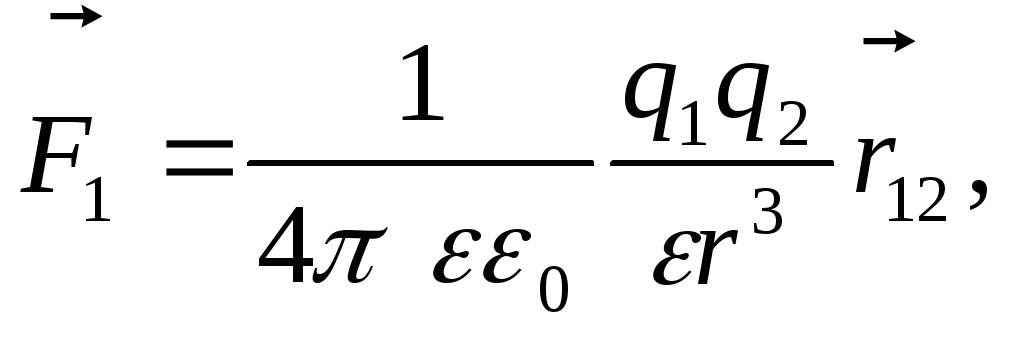
where 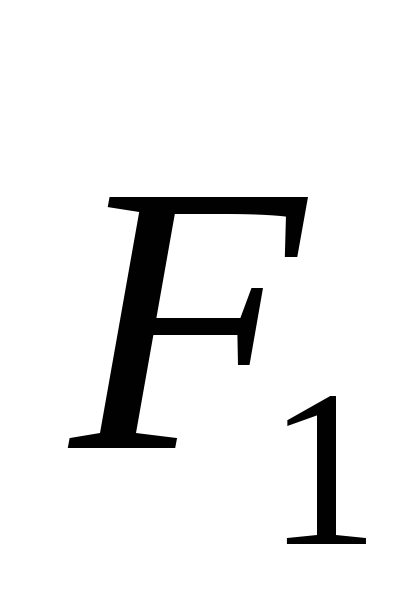 -vector forces acting on charge
-vector forces acting on charge 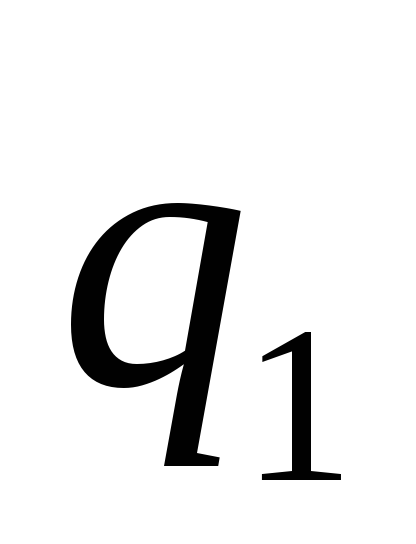 from charge
from charge
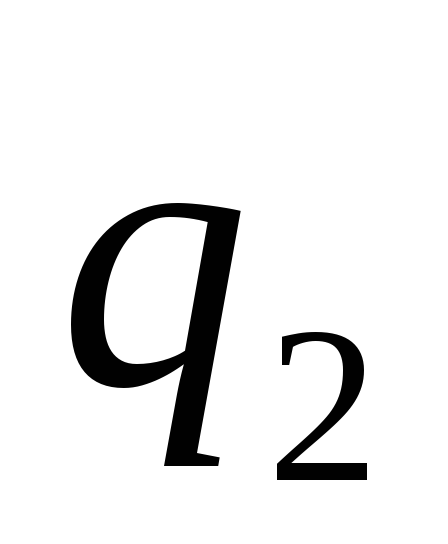 ,
,
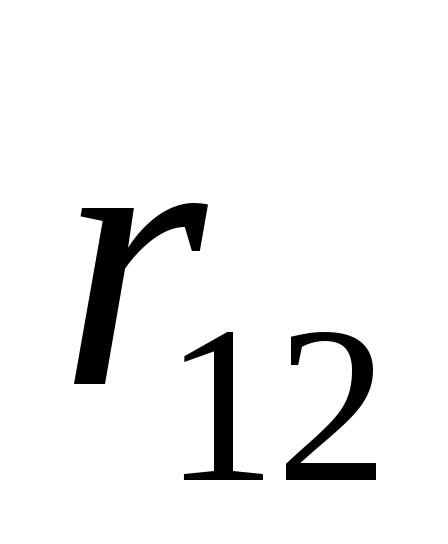
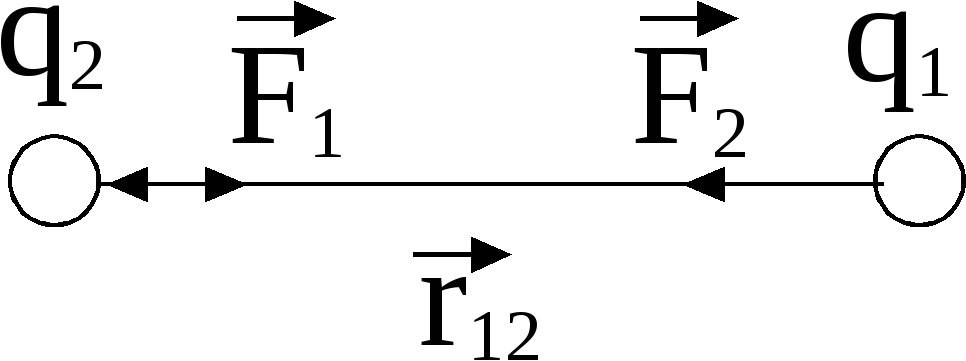 - radius-vector connecting charge
- radius-vector connecting charge 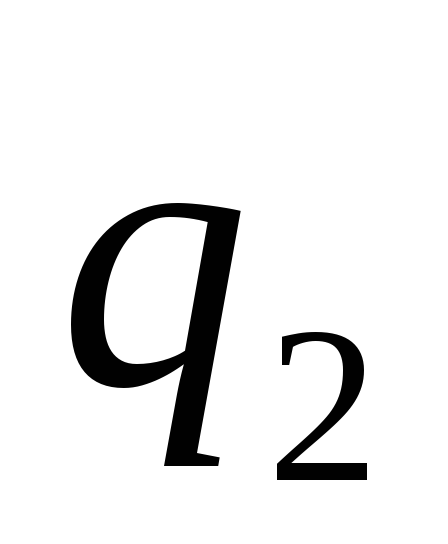 with charge
with charge
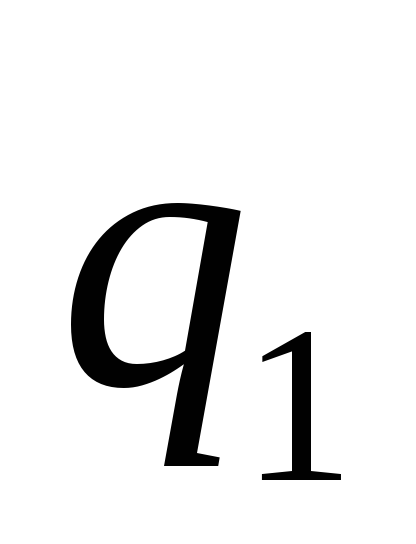
r. -Module radius-vector 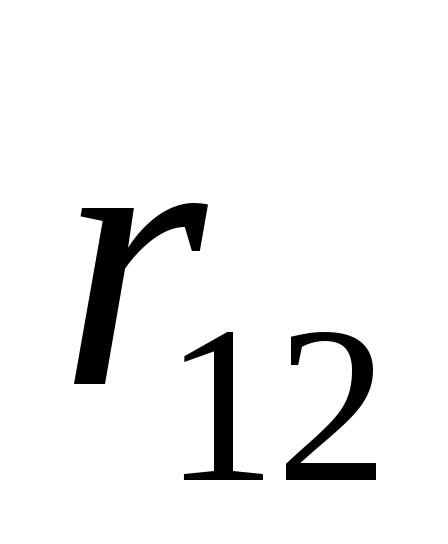 .
.
Any charged body consists of a variety of point electrical charges, so electrostatic force with which one charged body acts to another is equal to the vector sum of the forces applied to all point charges of the second body from each point charge of the first body.
1.3.Electrical field. Tension.
Space, in which the electrical charge is located, has certain physical properties.
On any other the charge introduced into this space is operated by the electrostatic forces of the coulon.
If at each point is the strength, it is said that in this space there is a power field.
The field along with the substance is a form of matter.
If the field is stationary, that is, does not change in time, and is created by fixed electrical charges, then such a field is called electrostatic.
Elektrostatics studies only electrostatic fields and interactions of fixed charges.
For characteristics electric field Enter the concept of tension
.
Tensionat every point of the electric field is called vector 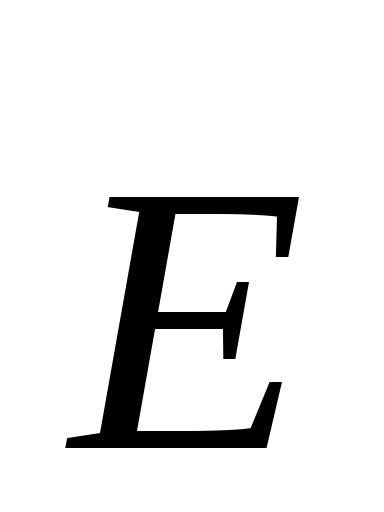 , numerically equal to the ratio of power with which this field acts on a test positive charge, placed at this point, and the magnitude of this charge, and aimed towards the action of force.
, numerically equal to the ratio of power with which this field acts on a test positive charge, placed at this point, and the magnitude of this charge, and aimed towards the action of force.
Trial chargewhich is entered in the field, is assumed point and often called a test charge.
- He does not participate in the creation of the field, which is measured with it.
It is assumed that this charge does not distort the studied field, That is, it is small enough and does not cause the redistribution of charges creating the field.
If on a trial point charge 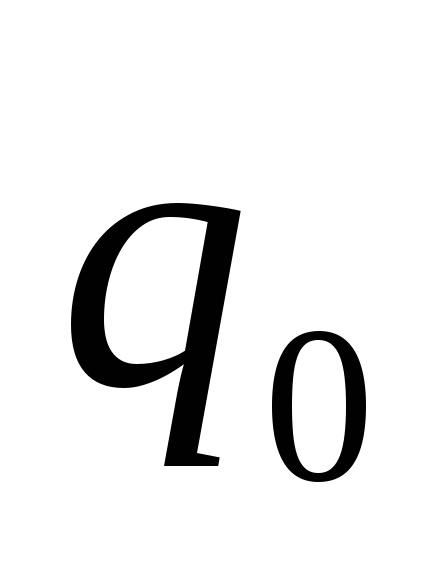 the field acts power
the field acts power 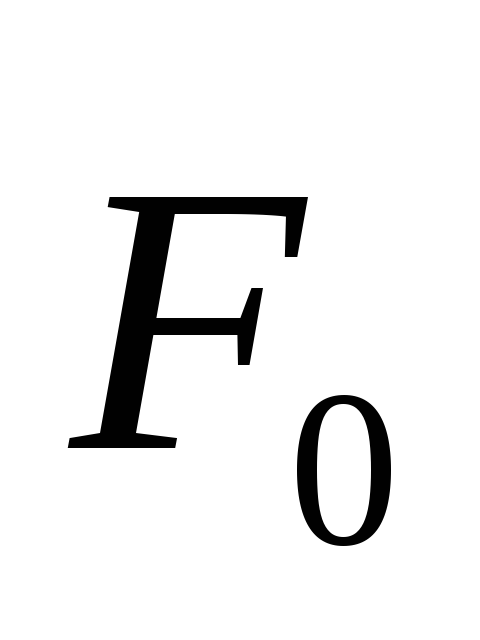 , Tension
, Tension 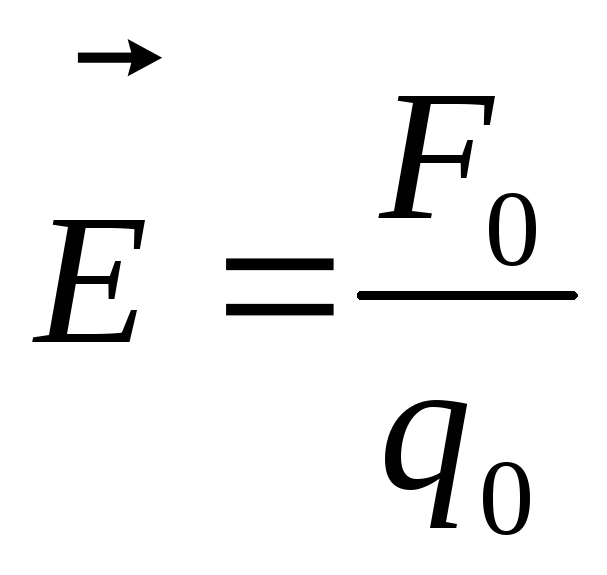 .
.
Units of tension:
C: 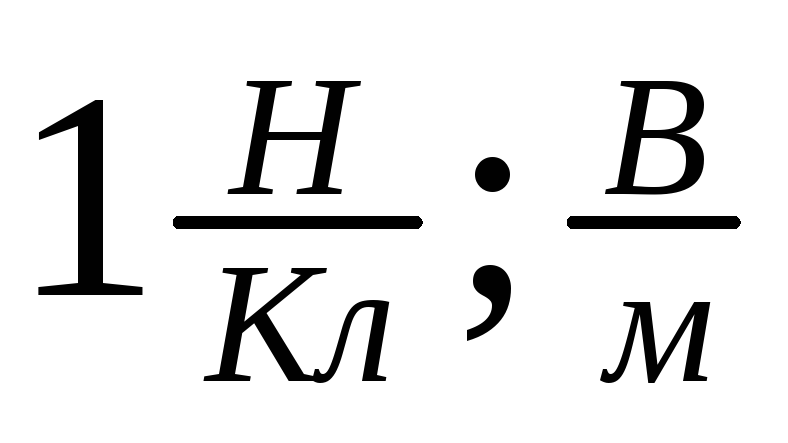
SGSE: 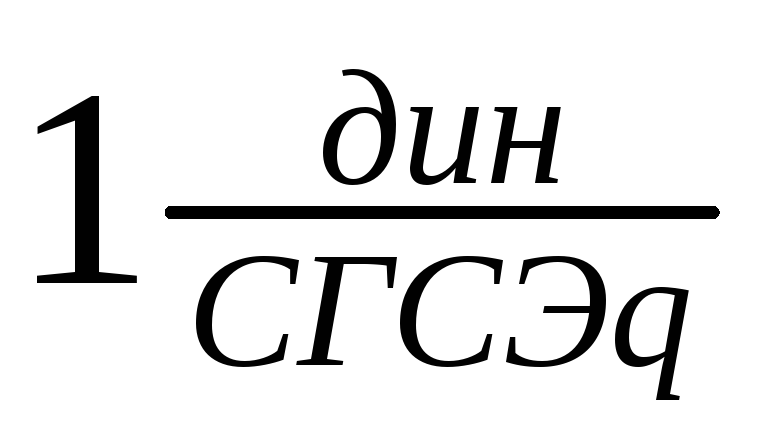
In the system S. expression
for  fields of point charge:
fields of point charge:
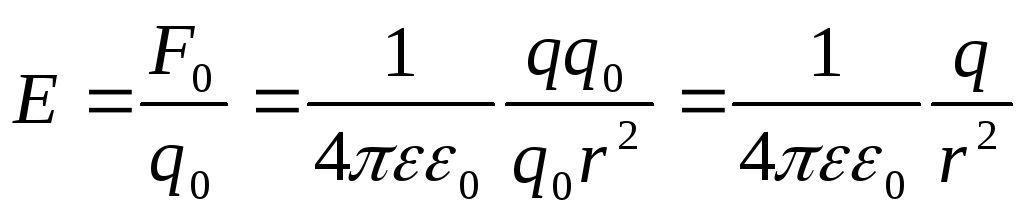 .
.
In vector form: 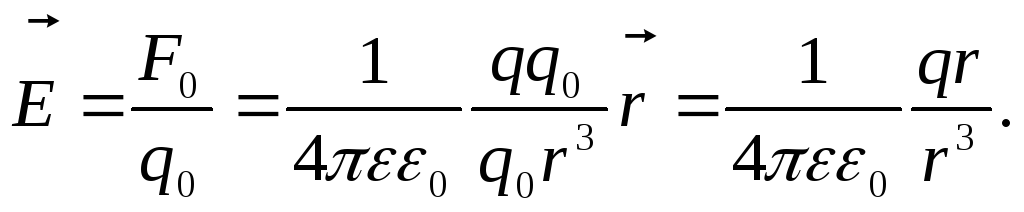
Here  - radius vector spent from charge q. creating a field at this point.
- radius vector spent from charge q. creating a field at this point.
T. 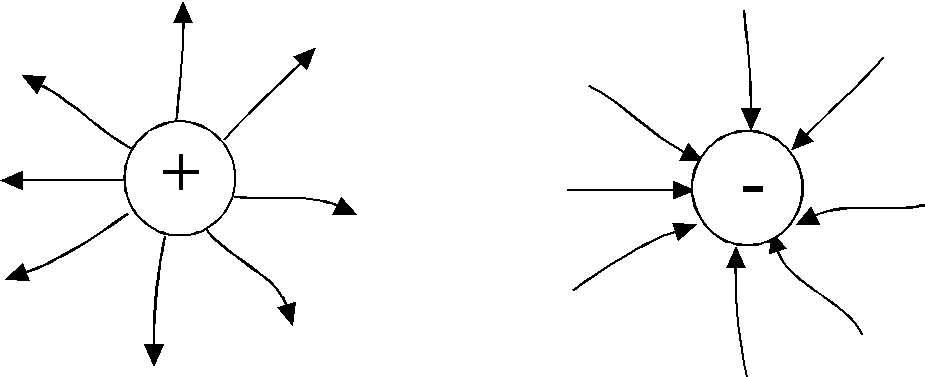 how point Dot Charge Electric Field Tension Vectorsq.
all points of the field are directed radially (Fig.1.3)
how point Dot Charge Electric Field Tension Vectorsq.
all points of the field are directed radially (Fig.1.3)
- from charge, if it is positive, "source"
- And to the charge, if he is negative "Stock"
For graphic interpretation Electric field are introduced concept of power line orthread lines . it
curve , tangent at each point to which coincides with the tension vector.
The line of tension begins on a positive charge and ends on a negative.
The line of tension does not intersect, since at each point of the field, the vector of tension has only one direction.
The law of Kulon. - This is a law describing the strengths of interaction between point electrical charges.
Charlel pendant was opened in 1785. After conducting a large number of experiments with metal balls, Charles Pendant gave such a wording of the law:
The module of the interaction force of two point charges in a vacuum is directly proportional to the product of the modules of these charges and inversely proportional to the square of the distance between them
Otherwise: two point charges in vacuum act on each other with the forces, which are proportional to the product of the modules of these charges, are inversely proportional to the square of the distance between them and are directed along the straight line connecting these charges. These forces are called electrostatic (Coulomb).
It is important to note that in order for the law to be faithful, it is necessary:
- the discretion of charges - that is, the distance between the charged bodies is much larger than their sizes - however, it can be proved that the strength of the interaction of two volumetric charges with spherically symmetric non-volatile spatial distributions is equal to the interaction of two equivalent spot charges placed in spherical symmetry centers;
- their immobility. Otherwise, additional effects come into force: the magnetic field of the moving charge and the corresponding additional force of Lorentz, acting on another moving charge;
- interaction in vacuum.
However, with some adjustments, the law is also fair for charge interactions in the environment and for moving charges.
In vector form in the wording of S. Kulon, the law is written as follows:
where - the force with which the charge 1 is valid for charge 2; - the magnitude of charges; - radius vector (vector directed from charge 1 to charge 2, and equal, module, distance between charges -); - proportionality coefficient. Thus, the law indicates that the same charges are repelled (and the relevant - attract).
Coefficient k.
In SGSE, the charge unit is chosen in such a way that the coefficient k. equal to one.
In the international system of units (s) one of the main units is the unit of force of an amper electric current, and the charge unit is a pendant - derived from it. The amount of ampere is defined in such a way that k. \u003d C2 · 10-7 Gn / m \u003d 8.9875517873681764 · 109 N · m2 / CL2 (or F-1 · m). In si coefficient k. Recorded in the form:
where ≈ 8,854187817 · 10-12 f / m - electrical constant.
In a homogeneous isotropic substance, the relative dielectric permeability of the medium ε is added to the formula denominator.
Culon law in quantum mechanics
In quantum mechanics, the law of the coulon is formulated not with the help of the concept of force, as in classical mechanics, and with the help of the concept potential energy Coulomb interaction. In the case when the system covered in quantum mechanics contains electrically charged particles, the components expressing the potential energy of the Coulomb interaction expressing the potential energy of the Coulomb interaction are added to the system of the Hamiltonian system, as it is calculated in classical mechanics.
So, the operator of the Hamilton atom with the charge of the kernel Z. It has the form:
j) \\ FRAC (E ^ 2) (R_ (ij)) "src \u003d" http://upload.wikimedia.org/math/d/0/8/d081b99Fac096B0E0C5B4290A9573794.png "\u003e.
Here m. - Electron mass, e. - His charge - the absolute value of the radius-vector j.- Electron, ![]() . The first term expresses kinetic energy electrons, the second term - the potential energy of the Coulomb interaction of electrons with the nucleus and the third is the term - the potential Coulomb energy of the mutual repulsion of electrons. The summation in the first and second term is carried out on all n electrons. In the third term, the summation goes through all pairs of electrons, and each pair occurs once.
. The first term expresses kinetic energy electrons, the second term - the potential energy of the Coulomb interaction of electrons with the nucleus and the third is the term - the potential Coulomb energy of the mutual repulsion of electrons. The summation in the first and second term is carried out on all n electrons. In the third term, the summation goes through all pairs of electrons, and each pair occurs once.
Cool law from the point of view of quantum electrodynamics
According to quantum electrodynamics, the electromagnetic interaction of charged particles is carried out by exchanging virtual photons between particles. The principle of uncertainty for time and energy allows the existence of virtual photons at the time between the moments of their emission and absorption. The smaller the distance between the charged particles, the mortar time you need to virtual photons to overcome this distance and therefore, the greater the energy of virtual photons is allowed by the principle of uncertainty. At low distances between charges, the principle of uncertainty allows for the exchange of both long-wave and short-wave photons, and at large distances, only long-wave photons participate in the exchange. Thus, with the help of quantum electrodynamics, the law of the coulon can be derived.
History
For the first time to explore the experimental law of interaction of electrically charged bodies offered G. V. Richman in 1752-1753. He intended to use the electrometer-"pointer" designed to this. The implementation of this plan was prevented by the tragic death of Richmana.
In 1759, Professor of Physics of the St. Petersburg Academy of Sciences F. Epinus, who took the Department of Richmana after his death, first suggested that the charges should interact inversely in proportion to the square square. In 1760, a brief message was appeared that D. Bernoulli in Basel set a quadratic law using the electrometer constructed. In 1767, they were attracted in its "History of Electricity" noted that the experience of Franklin, who found the absence of an electric field inside the charged metal ball may mean that "Electrical attraction should be exactly the same law as a lot, that is, the square of the distance". Scottish physicist John Robison argued (1822) that in 1769 discovered that the balls with the same electric charge are repelled with the force inversely in a proportional square of the distance between them, and thus anticipated the opening of the Culon law (1785).
Approximately 11 years before Coulomb, in 1771, the law of interaction of charges was experimentally opened by G. Cavendis, but the result was not published and for a long time (over 100 years) remained unknown. Manuscripts Cavendish were awarded to D. K. Maxwell only in 1874, one of the descendants of Cavendish at the solemn opening of the Cavendish laboratory and published in 1879.
The pendant himself was engaged in the study of the tight of the threads and invented the tweaks. He opened his law, measuring with the help of them the strength of the interaction of charged balls.
The law of Kulon, the principle of superposition and the Maxwell equation
The law of the coulon and the principle of superposition for electric fields are fully equivalent to the Maxwell equations for electrostatics ![]() and. That is, the law of the coulon and the principle of superposition for electric fields are performed if and only if Maxwell equations for electrostatics are performed and, on the contrary, the Maxwell equations for electrostatics are performed if and only if the Culon law and the principle of superposition for electric fields are performed.
and. That is, the law of the coulon and the principle of superposition for electric fields are performed if and only if Maxwell equations for electrostatics are performed and, on the contrary, the Maxwell equations for electrostatics are performed if and only if the Culon law and the principle of superposition for electric fields are performed.
The accuracy of the accuracy of the law of Culon
The law of Cool is an experimentally established fact. His justice has been repeatedly confirmed by more accurate experiments. One of the directions of such experiments is to check whether the indicator is distinguished r. In the law from 2. To search for this difference, the fact that if the degree is exactly equal to two, the field inside the cavity in the conductor is missing, Whatever the form of the cavity or conductor.
Experiments conducted in 1971 in the United States E. R. Williams, D. E. Folller and G. A. Hill, showed that the degree in the law of the coulon is 2 with an accuracy of it.
To verify the accuracy of the Culon law on intraatomic distances of W. Yu. Lamb and R. Rutinford in 1947, measuring the relative location of hydrogen energy levels were used. It was found that at the distances of the order of atomic 10-8 cm, the indicator of the degree in the law of the coulon differs from 2 no more than 10-9.
The coefficient in the law of the coulon remains constant up to 15 · 10-6.
Amendments to the law of the coulon in quantum electrodynamics
At small distances (the order of the computers' wavelength of the electron, ≈3.86 · 10-13 m, where - the mass of the electron, is a constant plank, - the speed of light) become essential nonlinear effects of quantum electrodynamics: the generation of virtual electron positron (A is imposed on the exchange of virtual photons (and Also muon-antimuonny and ton-anti-anti-nitonic) pairs, and also decreases the effect of shielding (see renormalization). Both effects lead to the appearance of exponentially decreasing member of the order in the expression for the potential energy of the interaction of charges and, as a result, to an increase in the interaction force compared to the Culon calculated by the law. For example, the expression for the potential of a point charge in the SGS system, taking into account the radiation amendments of the first order takes the form:
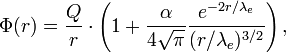
where - the Compton wavelength of the electron, is a thin structure constant and. At distances of about ~ 10-18 m, where - the mass of the W-boson, there are already electrical effects into the game.
In strong outdoor electromagnetic fieldsconstituting a noticeable share of vacuum breakdown field (order ~ 1018 V / m or ~ 109 TLs, such fields are observed, for example, near some types of neutron stars, namely the magnets) the law of the coulon is also violated by the Delbryukovsky scattering of metropolis photons on the photons of the external field and other, more complex nonlinear effects. This phenomenon reduces Coulomb force not only in micro- and in macroscopab, in particular, in a strong magnetic field, the Coulomb potential is not falling inversely in proportion to the distance, and exponentially.
Cool law and Vacuum polarization
The phenomenon of vacuum polarization in quantum electrodynamics is to form virtual electron-positron pairs. The cloud of electron-positron pairs shields the electrical electrical charge. Screening grows with an increase in the distance from the electron, as a result, an efficient electrical electron charge is a decreasing distance function. The effective potential created by an electron with an electrical charge can be described by the dependence of the species. Effective charge depends on the distance by logarithmic law:
- T. N. permanent structure ≈7.3 · 10-3;
- T. N. Classic electron radius ≈2.8 · 10-13 cm.
Effect of Yulling
Disable phenomenon electrostatic potential Spot charges in vacuo from the value of the Culon law is known as the effect of the Yulling, which for the first time calculated deviations from the Culon law for the hydrogen atom. The effect of Yulling gives an amendment to the Lamb shift 27 MHGs.
Cool law and super heavy nuclei
In a strong electromagnetic field near the super heavy nuclei with charge 170 "src \u003d" http://upload.wikimedia.org/math/0/d/d/7/0D7B5476A5437D2A99326CF04B131458.png "\u003e a vacuum is restructuring similar to the usual phase transition. This leads to amendments To the law of the coulon.
The significance of the law of Culon in the history of science
The law of Coulomb is the first open quantitative and formulated on the mathematical language by law for electromagnetic phenomena. From the opening of the law of Kulon began modern science About electromagnetism.
The interaction of electrical charges is described by the Culon law, which claims that the strength of the interaction of two resting point charges in a vacuum is equal to
where the value is called an electrical constant, the dimension of the value is reduced to the dimension rate to the dimension electrical capacity (Farad). Electric charges There are two types that conditionally call a positive and negative. As experience shows, the charges are attracted if they are different and repel if the same names.
In any macroscopic body, there is a huge amount of electrical charges, since they are part of all atoms: electrons are negatively charged, protons included in the atomic nuclei are positive. However, the majority of the bodies with whom we are dealing are not charged, since the number of electrons and protons included in the atoms is equally, and their charges in absolute value are accurately coincided. However, the bodies can be charged, if you create an excess or disadvantage of electrons in them compared to protons. To do this, it is necessary to transfer electrons that are part of some body, another body. Then the first lack of electrons and, accordingly, positive charge, the second is negative. This kind of processes occur, in particular, with friction of tel about each other.
If the charges are in some medium that occupies all the space, the strength of their interaction is weakened compared with the strength of their interaction in vacuum, and this weakening does not depend on the values \u200b\u200bof charges and the distance between them, and depends on the properties of the environment. The characteristic of the medium that shows how many times the strength of the interaction of charges in this medium is weakened compared with the strength of their interaction in vacuum, is called the dielectric constant of this medium and, as a rule, is indicated by the letter. Cool formula in medium with dielectric constant takes
|
If there are no two, and a larger number of point charges to find the forces operating in this system, a law is used, which is called principle superposition 1. . The superposition principle claims that in order to find a force acting on one of the charges (for example, for a charge) in a system of three point charges, and the following should be done. First we need to mentally remove the charge and according to the Culon law to find the power acting on the charge from the side of the remaining charge. Then you should remove the charge and find the power acting on the charge from the charge side. Vector sum of the forces and will give a desired force.
The principle of superposition gives a recipe for the search for the interaction force of internal charged bodies. It should mentally split every body on the parts that can be considered point, according to the law of the coulon, find the power of their interaction with point parts, which is broken down by the second body, sum up the resulting vector. It is clear that such a procedure is mathematically very complex, if only because it is necessary to add the infinite number of vectors. In mathematical analysis, methods of such summament have been developed, but they are not included in the school course of physics. Therefore, if such a task will meet, then the summation in it should be easily implemented on the basis of certain considerations of symmetry. For example, from the described summation procedure it follows that the force acting on the point charge placed in the center of a uniformly charged sphere is zero.
In addition, the schoolboy must know the (without output) of the formula for the force acting on the point charge from the side of the uniformly charged sphere and infinite plane. If there is a radius sphere, evenly charged with charge, and a point charge, located at a distance from the center of the sphere, then the value of the interaction force is equal
if the charge is inside (and not necessarily in the center). From formulas (17.4), (17.5) It follows that the sphere outside creates the same electric field As a whole charge, placed in the center, and inside - zero.
If there is a very large plane with an area of \u200b\u200buniformly charged charge, and the point charge, the strength of their interaction is equal
|
where the amount is 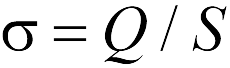 has the meaning surface density Charge plane. As follows from formula (17.6), the strength of the interaction of a point charge and the plane does not depend on the distance between them. Pay attention to the reader to the fact that Formula (17.6) is approximate and "works", the more accurately, the further point charge is from its edges. Therefore, when using formula (17.6), it is often said that it is valid in the framework of neglecting "edge effects", i.e. When the plane is considered an endless.
has the meaning surface density Charge plane. As follows from formula (17.6), the strength of the interaction of a point charge and the plane does not depend on the distance between them. Pay attention to the reader to the fact that Formula (17.6) is approximate and "works", the more accurately, the further point charge is from its edges. Therefore, when using formula (17.6), it is often said that it is valid in the framework of neglecting "edge effects", i.e. When the plane is considered an endless.
Consider now the data solution in the first part of the book of tasks.
According to the Culon law (17.1), the value of the interaction force of two charges from tasks 17.1.1 Formula is expressed
|
Charges repel (answer 2 ).
Since water drip from tasks 17.1.2 Has a charge  (- The proton charge), then it has an excess of electrons compared to protons. It means with the loss of three electrons, their excess will decrease, and the charge of the droplets will become equal
(- The proton charge), then it has an excess of electrons compared to protons. It means with the loss of three electrons, their excess will decrease, and the charge of the droplets will become equal  (answer 2
).
(answer 2
).
According to the Coulomb law (17.1), the value of the interaction force of two charges with an increase in times of distances between them will decrease at times ( task 17.1.3 - answer 4 ).
If the charges of two point bodies increase at times with a constant distance between them, the strength of their interaction, as follows from the Culon law (17.1), will increase at times ( task 17.1.4 - answer 3 ).
With an increase in one charge, 2 times, and the second in 4, the numerator of the Culon law (17.1) increases 8 times, and with an increase in distance between charges 8 times - the denominator increases 64 times. Therefore, the power of the interaction of charges from tasks 17.1.5 decrease 8 times (answer 4 ).
When filling the space by a dielectric medium with dielectric constant \u003d 10, the power of charge interaction according to the Coulomb Act (17.3) will decrease in 10 times ( task 17.1.6 - answer 2 ).
The strength of Coulomb interaction (17.1) acts both on the first and the second charge, and since their masses are the same, then acceleration of charges, as follows from the second law of Newton, at any time the same ( task 17.1.7 - answer 3 ).
A similar task, but the bulbs are different. Therefore, with the same force, the ball accelerates with a smaller mass 2 times greater than the acceleration of the ball with a smaller mass 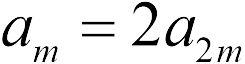 , and this result does not depend on the values \u200b\u200bof the charges of balls ( task 17.1.8. - answer 2
).
, and this result does not depend on the values \u200b\u200bof the charges of balls ( task 17.1.8. - answer 2
).
Since the electron is charged negative, it will be repelled from the ball ( task 17.1.9). But since the initial speed of the electron is directed towards the ball, it will move in this direction, but its speed will decrease. At some point he will stop for a moment, and then move from a ball with increasing speed (answer 4 ).
In the system of two charged balls related to the thread ( task 17.1.10) There are only internal forces. Therefore, the system will rest and to find the strength of the tension of the thread, the equilibrium conditions can be used. Since only the Coulomb force and the force of tensioning the thread are valid for each of them, then we conclude from the equilibrium condition that these forces are equal in magnitude.
This magnitude will be equal to the force of tension of the threads (answer 4 ). Note that the consideration of the equilibrium condition of the central charge would not help find the power of tension, but would lead to the conclusion that the tension forces are the same (however, this conclusion is so obvious due to the symmetry of the task).
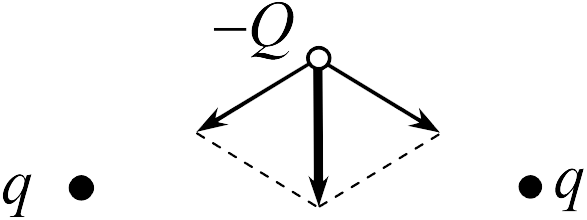 To find the strength acting on the charge - in task 17.2.2., We use the principle of superposition. For the charge - there are the forces of attraction to the left and right charges (see Figure). Since the distance from the charge - before charges the same, the modules of these forces are equal to each other and they are directed at the same angles to the straight line, connecting the charge - from the middle of the segment. Therefore, the force acting on the charge is directed vertically down (vector of the resulting force is highlighted in fat in the figure; answer 4
).
To find the strength acting on the charge - in task 17.2.2., We use the principle of superposition. For the charge - there are the forces of attraction to the left and right charges (see Figure). Since the distance from the charge - before charges the same, the modules of these forces are equal to each other and they are directed at the same angles to the straight line, connecting the charge - from the middle of the segment. Therefore, the force acting on the charge is directed vertically down (vector of the resulting force is highlighted in fat in the figure; answer 4
).
(answer 3 ).
From formula (17.6), we conclude that the correct answer in task 17.2.5. - 4 . IN task 17.2.6 It is necessary to use the formula for the interaction force of a point charge and sphere (formula (17.4), (17.5)). We have \u003d 0 (answer 3 ).