Dielectrics in the electric field do not behave as the conductor, although they have something in common. Dielectrics differ from the conductors in that they do not have free chargers of charges. Still, they are there, but in very small quantities. In conductors, such carriers of charges are electrons freely moving along the crystal metal grid. But in dielectrics, electrons are firmly connected with their atoms and cannot move freely.
When making a dielectric to the electric field, it comes to electrification as well as in the conductor. The difference between dielectrics is that electrons cannot freely move in volume as it happens in the conductors. But under the action of external electric field Inside the dielectric substance molecules, some charge shift appears. Positive shifts along the direction of the field, and negative against. As a result, the surface receives a certain charge. The process of education of charge on the surface of dielectrics under the action of the electric field is called polarization of dielectric.
All dielectrics are divided into two categories. Dielectrics belonging to the first category have molecules that even in the absence of an external electric field form dipoles. They're called polar . The polar dielectrics include water ammonia acetone and ether. The dipoles of such dielectrics in the absence of the field are chaotic due to the heat movement. And, consequently, the charge on the surface of such a substance is zero.
But when it is introduced into the external electric field, the dipole is there a molecule seek to turn around along the field. It turns out that the positive charge of the previous dipole looks at the negative next. Consequently, they compensate each other. But the dipoles located near the surface itself is not a couple. Thus, uncompensated related charges are formed on the surface of the material. On the one hand positive with the other negative. But this prevents the thermal movement of molecules.
Figure 1 - polarization of polar dielectric
The second category of dielectrics are those in which there are positive and negative charges inside the molecule. But they are so close to each other that their effect is mutually compensated. But when making such a molecule in the charge field will be shown for some distance. Thus, the dipole is formed. At such molecules do not affect the thermal motion and, therefore, the polarization in them does not depend on temperature.
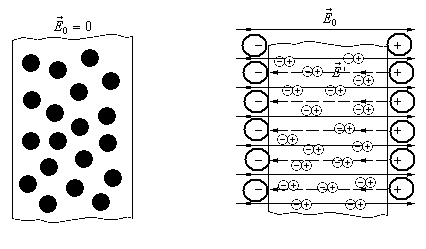
Figure 2 - Polarization of non-polar dielectric
Charges on the surface of dielectrics, in contrasts of charges induced in conductors, cannot be separated from the surface. When removing the electric field, the polarization will disappear. The charges are redistributed again in the volume of substance.
The intensity of the field can not be enlarged infinitely. Since at a certain amount of charges will be shown so much that the structural change in the material will occur, simply speaking, the sample of the dielectric. In this case, it loses its insulating properties.
However, it should be emphasized that a closed hollow conductor shields cavity within himself only from external charges and fields. If you make charges inside the cavity, then the electric field appears there (despite the fact that in the conductor itself the field must still be zero).
where S is the absorption of induced charges (we assume that the conductor is generally uncharged).
In practice, it is necessary to solve the following task. Any external field is given. It introduces the conductor of the specified form. It is necessary to find the distribution of charges induced on it and those changes in the total field outside the conductor to which they lead. The density of charges at a given conductor potential is determined by the curvature surface: s is growing with an increase in positive curvature (bulges) and decreases with an increase in negative curvature (concavity).
If the surface of the conductor has depressions and bulges, then the surface density of charges will be different in different points of the surface of the conductor. Where there is a bulge, especially the point, the density of charges will be greater than where there is a buptine (see Fig. 2.6).
Fig. 2.6. Distribution of charges on the surface of the conductor of complex shape
In Figure 2.7, experience is shown to study the distribution of charges on the surface and in the volume of the conductor. Electric charge It is distributed over the surface of the conductor, and not in its volume. This is demonstrated using a metal bowl and two hemispheres, which can be surrounded. After the charge message, the electrometer shows the presence of charge on its surface. If you touch the ball to another ball made up of two hemispheres, then the charge is distributed between both balls. If you closure a charged ball inside the hemispheres, then the entire charge from the ball flows into the hemisphere, and after their opening, the electrowema shows the absence of a residual charge on the ball.
The density of charges on the surface of the complex form is different in different points of its surface: the greater the curvature of the surface, the higher the density of the charge. Let's touch the test metal ball of different parts of the surface of the conductor of the complex shape consisting of cylindrical and two conical surfaces, and then the contact of the electroometer. In this case, there is almost a complete absence of an electrometer arrow deviation after touching the ball of a concave body part, a small deviation after the touch of the cylindrical part and the maximum - when touched the isge of the convex part of the body.
Electric field in dielectric
§1 Conductors and dielectrics. Polar and non-polar molecules. Ionic crystals. Free and related charges. Types of polarization.
- Conductors and dielectrics see lection. 1 by electrostatics.
- Types of dielectrics.
The dielectric molecule, like the molecule of any other substance, is electrically neutral. This means that the total negative charge of electrons is equal to the total positive charge of the nuclei.
If the molecule in the absence of an external electric field centers of gravity of positive and negative charges coincide, that is, the dipole moment of the molecule, then such molecules are called notolar. These include moleculesH 2, O 2, N 2.
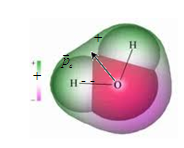
Molecules that in the absence of an external field centers of gravity positive and negative charges do not coincide, that is, there is a dipole moment, called polar. These includeH 2 O, CO, NH, HCl, SO 4, etc.
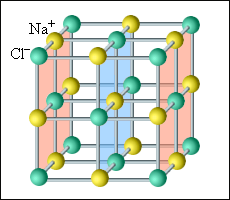
Ionic crystals (NaCl, KBR, KCL ) have a crystalline structure. In the nodes of the spatial lattice are located with alternating ions of different characters. In ionic crystals, individual molecules can not be isolated. They should be considered as a system of two sublattices - positive and negative.
Crystal grille
- Types of polarization.
Polarization The dielectric is called the process of orientation of dipoles or the appearance under the influence of the electric field oriented by the field of dipoles.
(The appearance of the dipole moment in the dielectric is called Polarization)
As a result of polarization, the molecule acquires a dipole moment, the value of which is proportional to the field
where α is the polarizability of the molecule (characterizes the "reaction" of the molecule to the electric field). Α - characteristics of an atom or ion.
As a magnitude characterizing the degree of polarization of the dielectric, vector is taken. Polarizedness- dipole moment Unit of volume (or density of the dipole moment)
where - the dipole moment of the same molecule, - the total dipole moment of volumeV..
Three types of dielectrics correspond to three types of polarization
Ion polarization - The occurrence of the dipole moment in ionic crystals caused by the displacement of the sublattices of positive ions along the field, and negative - against the field.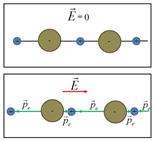
- Orientation (dipole) polarization - The occurrence of the dipole moment in a dielectric with polar molecules due to the orientation of the dipole moments of molecules in the direction of the field.
- Free and related charges
Charges that when applying an external electric field can freely move through the conductor, and are not associated with the ions of the crystal lattice, are called free.
Charges included in the molecule, which under the action of the external field only slightly shift from their equilibrium positions, and leave the limits of the molecule cannot be called connected.
§2 field strength in dielectric.
In isotropic dielectrics, the polarization vector linearly depends on the field strength
where χ is Dielectric perception Substances, shows how the dielectric reacts (perceives) to an external electric field.
α is the characteristics of a separate molecule (ion), χ - characteristics of the entire dielectric, that is, the characteristics of the substance as a whole. χ does not depend on both in weak fields. χ - dimensionless value
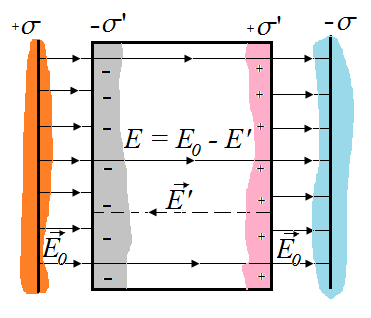 If there is a layer of dielectric between the plates of a flat capacitor, then as a result of polarization, positive charges in the dielectric will be shown along the field, and negative - against the field, and on the right face (in figure) there will be an excess of positive, and on the left grain - excess negative charges with surface density + Σ 'and -σ'. These charges will create a homogeneous field inside the dielectric plate, the tension of which by the Gauss theorem is equal to
If there is a layer of dielectric between the plates of a flat capacitor, then as a result of polarization, positive charges in the dielectric will be shown along the field, and negative - against the field, and on the right face (in figure) there will be an excess of positive, and on the left grain - excess negative charges with surface density + Σ 'and -σ'. These charges will create a homogeneous field inside the dielectric plate, the tension of which by the Gauss theorem is equal to
Where - the surface density of related charges.
Outside a dielectric. The external field and internal are directed towards each other, therefore, inside the dielectric
![]()
Outside a dielectric.
We define the surface density of related charges. Full dipole moment Plate dielectric
![]()
where S. - area of \u200b\u200bthe edge of the plate,d.- Her thickness. On the other hand, the full dipole moment is equal
Dielectrics call bodies that are not conductivecurrent current.
The term "dielectric" was introduced by M. Faraday to designate substances through which the electrical fields penetrate, in contrast to metals, within which there is no electrostatic field. Dielectricians include solid bodies, such as ebonite, porcelain, and liquids (for example, clean water) and gases.
When it changes external conditions (Heating, exposure to ionizing radiation, etc.) The dielectric can be carried out. The change in the state of the dielectric when placed in the electric field can be explained by its molecular maintenance. Conditionally allocate three grades of dielectrics: 1) polar;2) non-polar;3) crystal.
The first class belongs to such substances such as water, nitrobenzene, etc. Molecules of these dielectrics are not symmetrical, "mass centers" of their positive and negative charges do not coincide, therefore such molecules have an electric dipole moment even in the case when there is no electric field.
In fig. 12.19 schematically shows hydrochloric acid molecules ( but) and water (b)and the corresponding dipole moments in the debate 1. (1 Debu (D) - an out of system unit of the dipole moment of molecules: 1D \u003d 3.33564 10 -30 KL M.)
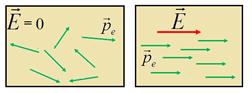
In the absence of an electric field, the dipole moments of molecules are oriented chaotic (Fig. 12.20, a) and the vector sum of the moments of all N.molecules is zero: 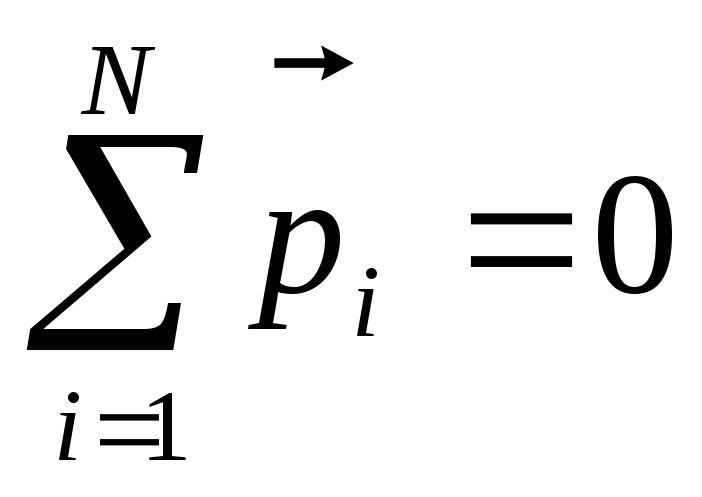
If the dielectric is placed in an electrical field, then the dipole moments of molecules seek to navigate along the field (Fig. 12.20, b)however, the full orientation will not be due to the molecular-thermal chaotic movement. In this case 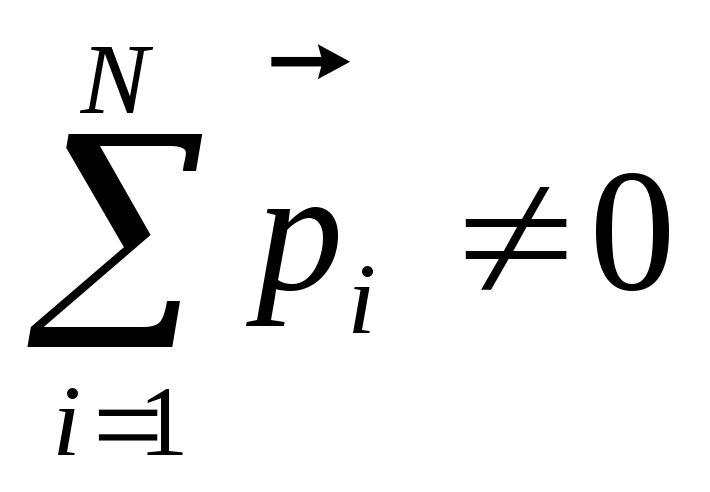
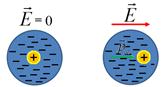
To the second class of dielectrics include substances (for example, hydrogen, oxygen, etc.), whose molecules do not have dipole moments in the absence of an electric field. In such molecules, the charges of electrons and nuclei are located so that the "centers of mass" of positive and negative charges coincide. If a non-polar molecule is placed in an electric field, then multi-way charges will be slightly shown in opposite sides and the molecule will have a dipole moment. In fig. 12.21 schematically in the form of circles showing such dielectric molecules in the absence of a field 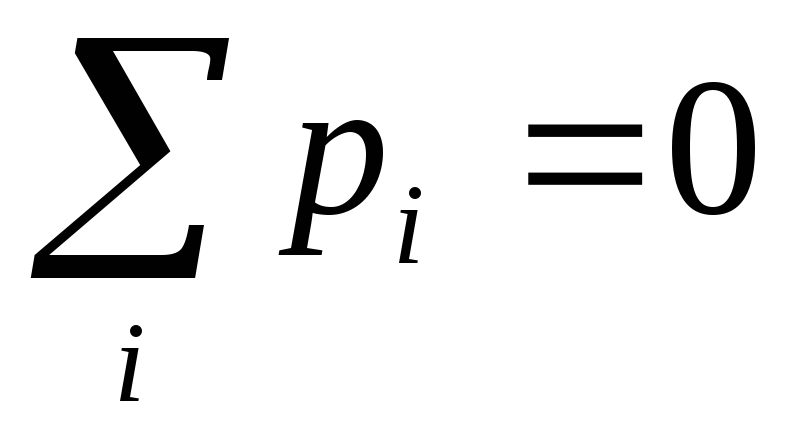 (but) and when the field is applied
(but) and when the field is applied 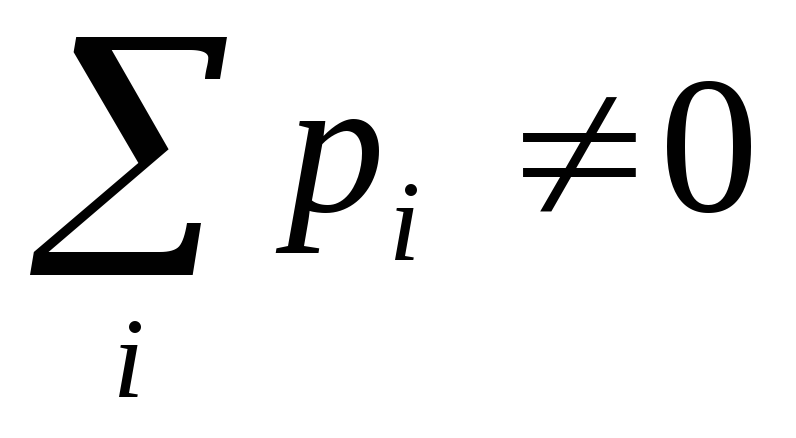 (b.) (Arrows in circles mean dipole moments of molecules).
(b.) (Arrows in circles mean dipole moments of molecules).
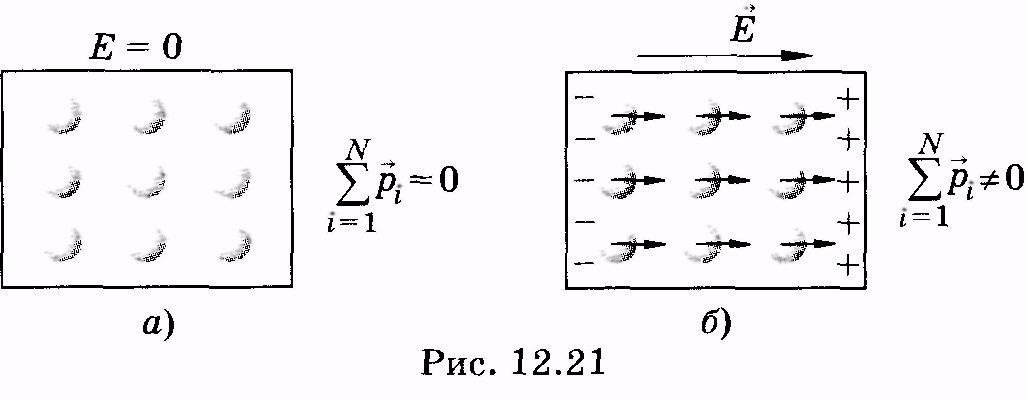
The third class is crystalline dielectrics (for example, NaCl), the grill of which consists of positive and negative ions. Such a dielectric can be schematically considered as a combination of two "sublattices", one of which is charged positively, the other is negative. In the absence of the field, the sublattice is located symmetrically and the total electric moment of such a dielectric is zero 1. (1 Strictly speaking, ionic crystals may have an electric moment and in the absence of an external field, but here it is not taken into account.) If a dielectric is placed in an electric field, then the sublattices will be shown a little in opposite sides and the dielectric will get an electric moment.
All these processes occurring in different dielectrics when applying an electric field are combined with a common term polarization,i.e. acquisition of a dielectric dipole moment.
For the first class of dielectrics is characteristic orientationalpolarization, for the second - electronici.e. displacement mainly electronic shells, for the third - ionic.Such a classification is conditional, since all types of polarization can simultaneously exist in a real dielectric.
The change in the electric field strength in which the dielectric is located, will affect the state of its polarization. Characterize the degree of polarization of the dielectric with the total electric moment of all it N.molecules 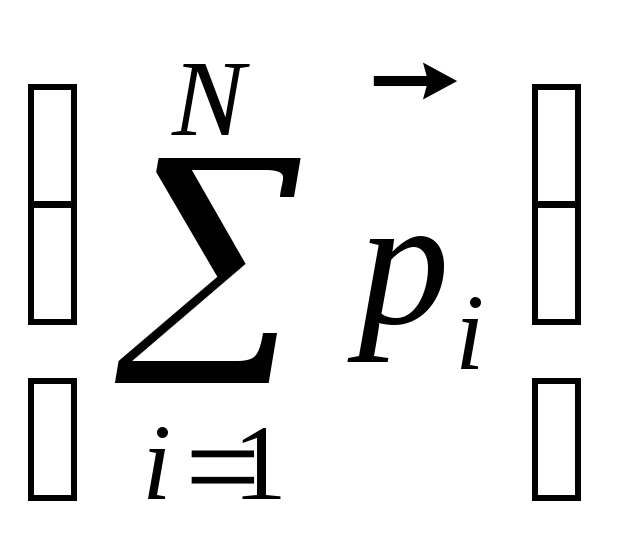 it is impossible, since this value depends, in particular, on the volume of the dielectric. For estimates of the state of the polarization of the dielectric introduced the value called polarizedthe mean value of which is equal to the ratio of the total electric moment of the volume element V.
dielectric to this volume:
it is impossible, since this value depends, in particular, on the volume of the dielectric. For estimates of the state of the polarization of the dielectric introduced the value called polarizedthe mean value of which is equal to the ratio of the total electric moment of the volume element V.
dielectric to this volume:
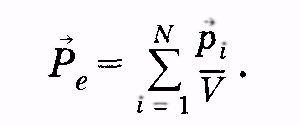 (12.36)
(12.36)
Unit of polarity is pendant per squaremeter(CL / m 2).
In the polarization of the dielectric on one of its surface (face), positive charges are created, and on the other - negative (see Fig. 12.20, b.and 12.21, b).These electrical charges are called associatedsince they belong to dielectric molecules (or crystal lattice With ion polarization) and can not move in the separation from molecules or be removed from the surface of the dielectric, in contrast to free charges, which are not in the perfect dielectric.
With an increase in the electric field strength, the degree of ordering of the orientation of molecules (orientation polarization) increases, the dipole moments of molecules (electronic polarization) increase, and there is also a larger displacement of the "sublattices" (ion polarization) - all this leads to an increase in the surface density of SV related electrical charges.
Thus, sv also characterizes the degree of polarization of the dielectric.
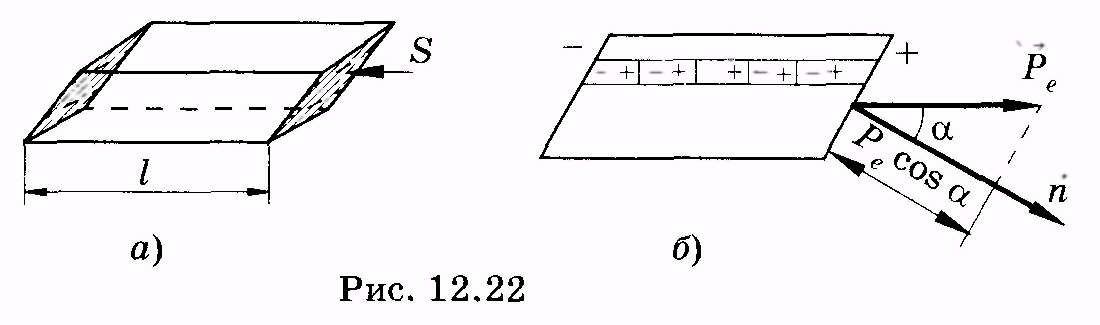
Install the connection between R e. and with an example of a polarized dielectric having a parallelepiped form (Fig. 12.22, but).Such a parallelepiped will be submitted as a totality of dipoles, which, simplicity, can be viewed as "chains"; One of them is shown in Fig. 12.22, b.Since the internal parts of the "chain" of dipoles are electrically compensated, then such a "chain" is similar to a long dipol with a distance between charges equal to the edge of the parallelepiped.
If the faces of the parallelepiped with an area s originatedtotal parallelepiped is numerically equal to dv /, but since 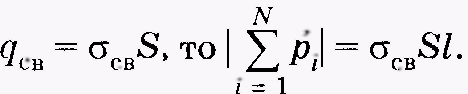 Parallelepiped volume V.
=
SI
cOS Based on the last two equalities we have
Parallelepiped volume V.
=
SI
cOS Based on the last two equalities we have
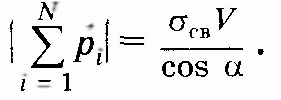 (12.37)
(12.37)
Considering (12.36) and (12.37), we get
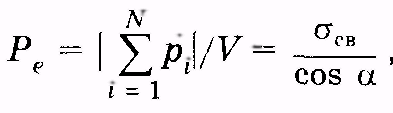
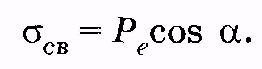
So, the surface density of the associated charges is equal to normal to the verge of vector component R e. .
R 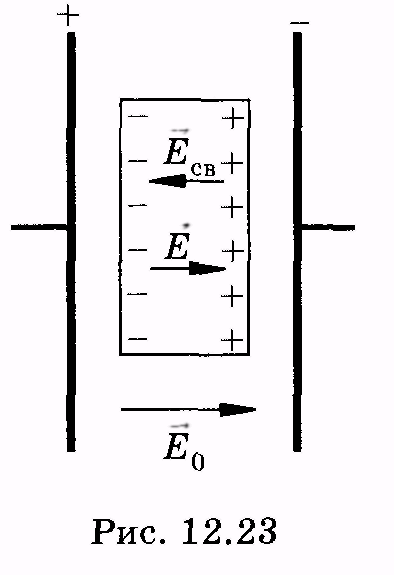 assemble, for example, a flat dielectric located in a homogeneous electric field (Fig. 12.23);
assemble, for example, a flat dielectric located in a homogeneous electric field (Fig. 12.23);  - field strength in the absence of dielectric (field in vacuo). Related charges create a homogeneous field intensity
- field strength in the absence of dielectric (field in vacuo). Related charges create a homogeneous field intensity  ,
as a result, the dielectric will be an electric field intensity
,
as a result, the dielectric will be an electric field intensity
E \u003d E. 0 - E. St. (12.39)
It is known that the dielectric constantthe environment is the attitude of the interaction force of charges in vacuo to the strength of the same charges at the same distance in the environment:
F. 0 / F. \u003d , or F. 0 = F..
Since the electric field strength is proportional to the power acting on the charge [cm. (12.1)], then a similar ratio can be recorded for E. 0 and E:
E. 0 = E.. (12.40)
The tension of the electric field formed by the associated electrical charges, E. SV \u003d sv / 0. For this example from (12.38) we have sv \u003d R e. , then E. SV \u003d. R e. / 0 . Substituting this formula and (12.40) in (12.39), we get E.= E. - R e. / 0 , or E.( 0 - 1) = =R e. / 0 , from
R e. = 0 (- 1)E.. (12.41)
As it was possible to expect, the polarity is proportional to the electric field strength in the dielectric. Based on (12.41), the concept is introduced dielectric perceivechiivostienvironments
= -1,(12-42)
which together S. dielectric permeability characterizes the ability of the dielectric to polarization and depends on its molecular structure, and possibly on temperature. In variables, electric fields vary also depending on the frequency.
In tab. 21 shows the values \u200b\u200bof dielectric constant for various biological media and some substances in a constant electric field at room temperature.
Table 21.
|
Egg protein | ||||
|
Vegetable oil | ||||
|
Blood whole | ||||
|
Gray substance of the brain | ||||
|
Milk cow |
Summer nerve | |||
|
White brain substance |
The difference between the dielectric constant of normal and pathological tissues and environments both in constant and variables, electric fields can be used for diagnostic purposes.
Usually, in the absence of an external electric field, dipole moments of dielectric molecules are or equal to zero (non-polar molecules), or erratically oriented (polar molecules). In both cases, the total dipole moment of the dielectric is zero.
Under the action of the external field, the dielectric polarizes, i.e. Acquires a different dipole moment \u003d, where - the dipole moment of one molecule. For a quantitative description of the degree of polarization of the dielectric use a vector value - polarity, defined as a dipole moment of a unit of dielectric volume:
and characterizing polarization at this point. Therefore, polarity can serve as a characteristic, both for an inhomogeneous external field and for an inhomogeneous dielectric.
In isotropic dielectrics with not very large values \u200b\u200bE polarity associated with field strength by the ratio
\u003d æE 0, (8.2)
where æ is a value that does not depend on the intensity called the dielectric susceptibility of the dielectric.
The dielectric susceptibility æ is the value of dimensionless, and always æ\u003e 0, and for most dielectrics (solid and liquid) is several units (although, for example, for alcohol æ 25, for water æ \u003d 80).
To establish the quantitative patterns of the field in the dielectric, we introduce into a homogeneous external electrostatic field with tension in a homogeneous external electrostatic field (it is created by two infinite parallel variemetically charged planes) a plate of a homogeneous isotropic dielectric, by placing it as shown in Figure 14. Under the action of the field, the dielectric polarizes, i.e. There is a shift of charges: positive shifts over the field, negative - against the field. As a result, on the right edge of the dielectric addressed to the negative plane, there will be an excess of a positive charge with a surface density of + S ¢, on the left - negative charge with the surface density -S. These non-compensated charges appearing as a result of the polarization of the dielectric are called connected. Since their surface density S ¢ is less than the density of S free charges of planes, then not the entire field is compensated by a dielectric charge field (number silest linespassing through the area of \u200b\u200bthe square in the dielectric and outside it is different due to the fact that part of the tension lines will pass through the dielectric, the other part is broken on the associated charges). Consequently, the polarization of the dielectric causes a decrease in fields in it compared to the initial external field. Outside the dielectric field strength is equal. Thus, the appearance of related charges leads to the emergence of an additional electric field (fields created by associated charges), which is directed against the external field (fields created by free charges) and weakens it. Resulting field strength inside the dielectric is equal
E \u003d E 0 - E. (8.3)
The field strength created by two infinite charged planes according to (6.5) E ¢ \u003d S ¢ / E 0, so
E \u003d E 0 -. (8.4)
We define the surface density of the associated charges S ¢. Full dipole moment of a dielectric plate P V \u003d PV \u003d PSD, where S is the area of \u200b\u200bthe plates, D is its thickness. On the other hand, the full dipole moment, according to (5.2), is equal to the product of the associated charges of each face of q ¢ \u003d s ¢ s for the distance D between them, i.e. P V \u003d S ¢ SD. Thus, PSD \u003d S ¢ SD, from where it follows that
i.e. the surface density of the associated charges S ¢ is equal to polarity R.
Substituting in (8.4) expressions (8.5) and (8.2), we obtain
E \u003d e 0 - æ,
where does the tension of the resulting field inside a homogeneous dielectric equal
E \u003d E 0 / (1 + æ) \u003d E 0 / E. (8.6)
Dimensional value
e \u003d 1 + æ (8.7)
it is called dielectric permeability of the medium. The dielectric permeability of the medium E shows how many times the field is weakened by a dielectric, characterizing the quantitative of the dielectric property to polarize in the electric field. Relation (8.6) in vector form:
Since the electric field strength in the dielectric decreases in e times, then it should be expected that all previously reviewed for vacuum physical quantities (for example, Coulomb force, dot charge field, point charge potential, potential interaction energy spot charges) Also decrease in e times. Accordingly, the relation for these quantities (2.1), (3.3), (4.9) and (4.7) in a solid, homogeneous, isotropic dielectric with dielectric constant E will take a look:
F \u003d k; E \u003d; j \u003d; W \u003d. (8.9)
Tasks of classical electrodynamics
Dielectrics of different types can be characterized by one common property: they have charged particles, but all of them are interconnected in such a way that they can not freely move over long distances. If dielectrics are in an electric field, then they, as they say, polarizes. At the same time, charges are moved at a distance of the distance between molecules.
Molecules from which dielectrics are electrically neutral. However, if they have no spherical symmetry, they have a dipole moment. Therefore, the behavior of the elementary dipole in the electric field is the basis for describing the electric field in the dielectric. Even if the molecule or atom has a spherical symmetry, in the electric field it disappears and the field-induced the dipole moment of the atom or molecule appears.
Gaseous and liquid dielectrics are isotropic (with the exception of liquid crystals), crystalline solid anisotropic bodies. Features of their behavior in electric fields We will also consider.
§29. Electric field dipole
Elementary dipole is two rigidly connected models and opposite to the charge sign at a distance. For its characterization, you can determine the vector of dipole moment
where - vector directed from a negative charge to a positive charge.
We define the potential of the electric field of the dipole (Rice29.1a):
![]() . (29.2)
. (29.2)
The final result corresponds to the potential at a high distance from the dipole (), which is at the beginning of the coordinates. The angle is the angle between the dipole moment vector and the radius-vector point in which the field is determined.
Electrical field on a large distance from the dipole will find by calculating the gradient of the potential:
![]()
![]() . (29.3)
. (29.3)
This field is shown in Figure 29.1b. Dotted - Equipotential () surface.
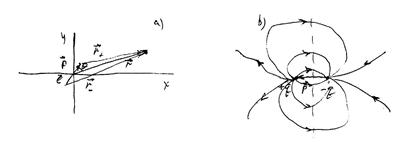
For a system of charges we can define the dipole moment of the system:
Moreover, the case of interest to us in dielectrics corresponds to the full charge to zero. In this case, the dipole moment will not depend on the choice of the coordinate system. Radius-vector charge in new system Coordinates is equal to where - a permanent vector. Vector dipole moment in the new coordinate system
it turns out the same.
§30 dipole in the external field
If you place an elementary dipole into a homogeneous electric field, then the resulting force acting on it will be zero. The moment of force will be different from zero in the general case:
Here is the radius-vector of a positive charge in the dipole - the radius-vector of the negative charge in the dipole.
The dipole in the electric field will focus under the action of this moment of forces until the field is oriented parallel.
In order to rotate the dipole oriented parallel to the field, at an angle, external forces must work
This work will be equal potential energy The dipole in the electric field, provided that the energy of the dipole oriented parallel field is selected for the beginning of the reference of the potential energy. If you choose another start of reference - the potential energy of the dipole is zero, the dipole energy in the electric field will be equal to:
If the electric field in which the dipole turned out to be inhomogeneous, then besides the moment of forces, it will not be equal to zero the resulting force. It will be equal to antigigode potential energy:
We will come to the same result, determining the forces acting on each dipole charge:
Now we can describe the behavior of the dipole in an external heterogeneous field. Under the action of the moment, it will be focused on the direction of the electric field (Fig. 30.1a), and retract to the region with greater field strength (Fig. 30.1b).

If the electric field creates another dipole, then we can determine the strength of their interaction
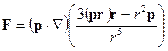 .
.
In particular, for parallel oriented dipoles on one straight line (Z axis) will be obtained
Diples are attracted with the force inversely proportional to the distance between them in the fourth degree.
§31 polarization of atoms and molecules
Let us turn from the description of abstract charges, dipoles to the description of real objects of nature - atoms and molecules, of which are all bodies, including dielectrics.
All atoms are symmetrical in the sense that the centers of gravity positive charges (They are all assembled in the nucleus of the atom) and negative charges (shell electrons) coincide. The dipole moment of the atom is zero. For molecules, two options are possible. For some symmetric molecules, the dipole moment is also zero (non-polar molecules). For example, hydrogen molecules, oxygen, carbon tetalist have no dipole moment.
Less symmetric molecules have a dipole moment (polar molecules). Molecule has a dipole moment ![]() . The water molecule seemingly should not have a dipole moment, but the axial symmetry in it is broken. The angle between the directions on the hydrogen atoms, the dipole moment of the water molecule.
. The water molecule seemingly should not have a dipole moment, but the axial symmetry in it is broken. The angle between the directions on the hydrogen atoms, the dipole moment of the water molecule.
In an electric field, even in symmetric atoms (molecules), a dipole moment appears, since the centers of gravity of positive and negative charges are shifted in opposite directions. This process is called the polarization of the atom. Since the mass of the electronic shell is less than the masses of the kernel, only the electronic shell has time in variable fields, and in a very short time with. Therefore, the polarization of the atom is sometimes called electron polarization.
In the first approximation, the discharge rate is proportional to the field in which the atom turned out to be, therefore, the induced (induced) dipole moment is also proportional to the field:
The coefficient of proportionality is called atomic polarizability.
It is interesting to trace its change for different atoms of the periodic system.
Atomic polarizability,
| Atom | H. | He. | LI | BE. | C. | Ne | Na. | AR | K. | Kr. | Xe. |
| 0.66 | 0.21 | 9.3 | 1.5 | 0.4 | 1.6 | 2.5 | 4.0 |
We see that atoms from the first group of elements in each row, the smallest - inert gases at the end of the row have the greatest polarizability. The easiest is polarized -electrons. With an increase in the number of completed shells, polarization increases, since the inner shells shield the kernel and the outer shell (valence electrons) is easier to polarizes. The study of the polarization of atoms gives important information about the structure of their electronic shells.
If we manage to fix the molecule that does not have spherical symmetry, its polarizability will be dependent on the mutual orientation of the electric field and the molecule. Naturally, in gases and conventional liquid dielectrics it is impossible to do this, but solid bodies that's quite possible.
In this case, the polarizability of the molecule will be set by the polarizability tensor. Then the dipole moment vector will be associated with the electric field strength vector by the ratio :. In general, the vector of the dipole moment and field strength is not parallel. For the simplest case of molecules with axial symmetry, such as oxygen molecules, in the coordinate system, where the z axis coincides with the axis of the molecule, there are only two differing components of the tensor. ![]() and (). The remaining components are zero.
and (). The remaining components are zero.
§32 Polarization of gaseous and liquid dielectrics
In gaseous and liquid dielectrics, the molecules are not fixed, they can rotate, move long intermolecular distances. If such dielectrics are in an electric field, then all dipole moments of molecules seek to navigate in the direction of the field. In a dielectric with spherically symmetric molecules (atoms of inert gases), the induced dipole moment of each molecule is always parallel to the field, since with chaotic movement with thermal speeds in collisions a disoriental moment restores its orientation in a very short time with. In a dielectric with non-polar molecules, which have no spherical symmetry, induced dipole moment due to their chaotic movement can be disorderent.
In a dielectric with polar molecules, their dipole moments seek to navigate through the field, collisions with other molecules with thermal motion will be disoriented with them. The average value of the projection of the dipole moment to the direction of the field for all dielectric molecules will not be zero - orientation polarization. In addition to orientational polarization, electronic polarization will occur, which is observed in any case.
Called electric susceptibility of dielectric.
Full polarity define after integration ranging from 0 to:
The electrical susceptibility of the polar gaseous dielectric will be:
This dependence is called Curie's law. With increasing temperature, the chaotic movement of molecules increases, electric susceptibility drops.
Gases and liquids consisting of polar molecules are isotropic media. When the electrical field is turned on, a minor anisotropy of properties appears due to the predominant orientation of dipoles in the direction of the electric field. However, she is quite small. In particular, to detect the anisotropy of the refractive index associated with it is very difficult, not to mention its practical use.
The world around us, however, is very diverse and among the fluids from the polar molecules there are such that in them, within the macro-volume with a characteristic size of ~ 10 μm, called domain, dipole moments are oriented parallel (liquid analogue of a solid segenelectric). We can talk about the dipole moment of the domain. In the absence of a field, the dipole moments of the domains are oriented arbitrarily, so that polarity is zero. When you turn on even a weak field, all domains are focused on the field, and we get gigantic polarity. The properties of such a fluid are substantially different in the direction of the field and in the direction of the perpendicular field. Such fluids are called liquid crystals.
The practical use of liquid crystals is quite wide. Consider their use in liquid crystal displays to display information.
The simplest use is based on the dependence of the reflection coefficient with a liquid crystal from the orientation of molecules in it. If they are not oriented, the incident light is partially absorbed, partially reflected from the surface. We see her gray. If all molecules in a small volume (pixel display) orient parallel surfaces, the light will almost completely absorb, there will be no reflection. This pixel will be perceived by us as a black spot. Similar displays are called passive because they work on the reflected light.
In active displays, a different property of liquid crystals is used: when the linearly polarized light wave is passed in the direction of polarity, the plane in which the vector fluctuates rotates. The thickness of the liquid crystal layer is chosen so that this rotation is at an angle. In the multi-layer display structure, the source light passes the first polarizer, becomes linearly polarized, passes through a layer of liquid crystal oriented in a constant field. In this case, the vector of the light wave turns at an angle, falls on the second crossed polaroid and emitted from the screen. In the color display, each pixel consists of three monochrome (red, green, blue), controlled independently. Monochrome light is obtained from white light source with light filters. In order to get a black point in a specific pixel, it is necessary to elaterate the liquid crystal in all three monochrome pixels using an additional (control) electrical field. Then the light through the second crossed polarizer will not pass. To obtain the gradation of each color, the control voltage on the pixel can be changed (usually with the help of a high-speed 8 bit DAC receive 256 levels of brightness of each color pixel). If the control voltage is less than the maximum, then the polarity of the liquid crystal does not disappear completely, the wicker plane of the light wave vector turns to the angle smaller and the light partially passes through the second crossed polarizer.
§33 Uniformly polarized isotropic dielectric
Consider an isotropic dielectric in a homogeneous electric field. For certainty, we assume that the field is created between the plane condenser plates, and almost all the space between them is filled with an isotropic dielectric thickness (Fig. 33.1).
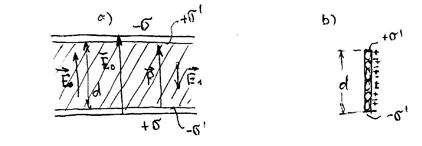
In the polarization of the dielectric dipoles are focused on the field. For a base with a base and height (Fig.33.1b), polarity is equal to:
![]() , (33.1)
, (33.1)
where is the dipole moment of one molecule. In addition, for simplicity, the foundation was taken in which one molecule is located. The polarity of the dielectric in this case will be homogeneous and equal surface density Related charges that appeared on the opposite margins of the plate.
Now we define the electric field in the dielectric:
it consists of a field created by charges on the plates, and the fields created by the associated charges on the dielectric surface. The direction of these fields is the opposite, therefore
In fig.33.1a, the field in the gap between the dielectric and the plate is equal, and the field in the dielectric is only less than :. The parameter is called dielectric constant.




